Early Outcomes of the Myval Octacor Transcatheter Heart Valve in Transcatheter Aortic Valve Implantation: A Single-Center Study From Serbia
© 2026 HMP Global. All Rights Reserved.
Any views and opinions expressed are those of the author(s) and/or participants and do not necessarily reflect the views, policy, or position of the Journal of Invasive Cardiology or HMP Global, their employees, and affiliates.
J INVASIVE CARDIOL 2026. doi:10.25270/jic/25.00264. Epub January 30, 2026.
Abstract
Objectives. Aortic stenosis (AS) is the most common valvular heart disease, with transcatheter aortic valve implantation (TAVI) now preferred for select severe cases. This study evaluated the early safety and performance of the new Myval Octacor Transcatheter Heart Valve (THV) (Meril Life Sciences), for which limited clinical data exist.
Methods. The authors retrospectively analyzed 43 consecutive patients with severe AS who underwent TAVI using the Myval Octacor THV at a tertiary cardiac center. Primary outcomes included all-cause mortality, stroke, major vascular complications, conduction abnormalities, new pacemaker implantation, paravalvular leak, and valve failure at 30 days follow-up.
Results. The median age was 81 years (IQR: 7), and 22 patients (51.2%) were female. Coronary artery disease was present in 24 (55.8%). Technical success rate was 100%. Mean pressure gradient decreased significantly post-procedure (55 mm Hg [IQR: 24] vs 5 mm Hg [IQR: 2]; P ≤ .001). No in-hospital deaths occurred. Two patients (4.7%) received permanent pacemakers during hospitalization, and 3 (7.0%) required pacemakers within a week because of conduction issues. Two patients (4.7%) experienced Bleeding Academic Research Consortium Type 3a bleeding. At 30 days, 42 patients remained in follow-up (98%), with 55% improved to New York Heart Association Class I. No vascular complications, stroke, acute kidney injury, valve thrombosis, or endocarditis occurred. One patient had valve failure from a significant paravalvular leak and 1 noncardiac death occurred 2 weeks post-discharge.
Conclusions. Early outcomes suggest that the Myval Octacor THV is a safe and effective option for TAVI in severe AS; however, larger studies with longer follow-up are required.
Introduction
Aortic stenosis (AS) is the most common valvular heart disease among older adults in developed countries, primarily resulting from progressive age-related calcific degeneration of the aortic valve.1 Surgical aortic valve replacement (SAVR) has traditionally been the gold standard for treating severe AS. However, transcatheter aortic valve implantation (TAVI) has emerged as an effective, less invasive alternative. Current American (American College of Cardiology [ACC]/American Heart Association [AHA]) and European (European Society of Cardiology [ESC]/ European Association for Cardio Thoracic Surgery [EACTS]) guidelines recommend TAVI for patients with severe, symptomatic AS across all surgical risk categories (Class I, Level A).2,3 Numerous studies have confirmed the noninferiority of TAVI compared with SAVR in high-, intermediate-, and low-risk patients, with comparable survival and improved functional outcomes.4-12 Despite this progress, vascular complications, conduction disturbances requiring pacemaker implantation, and paravalvular regurgitation remain important clinical challenges.13-15 Continuous advances in prosthesis technology and delivery systems aim to address these limitations.
Early real-world experience with the Myval Octacor Transcatheter Heart Valve (THV) (Meril Life Sciences) has been encouraging. By providing improved radial strength, precise deployment, and enhanced sealing, the Octacor expands the range of patients eligible for TAVI to those may not have been candidates for other devices. A multicenter Indian registry that included 123 patients reported 100% technical success, a 30-day mortality rate of 1.6%, and low rates of paravalvular leak (PVL).16 Similarly, the European OCTACOR-EU registry evaluated 252 patients treated across 15 centers and demonstrated 98.8% technical success, 1.2% 30-day mortality, and only 0.8% moderate-to-severe PVL.17 Despite these favorable outcomes, data from Eastern European populations remain scarce.
The present study contributes to the existing evidence by providing a detailed, single-center, real-world evaluation from Serbia, with comprehensive assessment of procedural performance and early clinical outcomes. In Serbia, the epidemiology of AS mirrors global trends, with an aging population driving an increasing demand for advanced transcatheter valve therapies. However, local experience with new-generation THVs is limited. As the leading tertiary cardiac centre performing TAVI in Serbia, our institution was the first to introduce the Myval Octacor THV, thereby expanding treatment options for patients across a broad range of anatomical and clinical presentations.
Methods
Study design
This retrospective, observational study examined all 43 consecutive patients diagnosed with severe AS who underwent TAVI using the Myval Octacor THV at our tertiary cardiac center from April to December 2023. The clinical data, which include demographic variables, medical history, familial predispositions, and physical examination findings, were obtained through history taking, systematic physical evaluations, and a comprehensive array of diagnostic modalities integral to the TAVI pathway.
Echocardiographic assessments were performed by 2 experienced echocardiographers in our department using standardized protocols, both immediately post-procedure and at 30-day follow-up. PVL was graded according to accepted criteria. Surgical risk was assessed using the Society of Thoracic Surgeons Predicted Risk of Mortality (STS-PROM) score, and patients were categorized as low (< 4%), intermediate (4%-8%), or high (> 8%) surgical risk.
Each patient’s eligibility for TAVI, determination of optimal vascular access, and selection of the most appropriate valve type were assessed by a multidisciplinary heart team comprising an interventional cardiologist, clinical cardiologist, echocardiographer, cardiac surgeon, anaesthesiologist, vascular surgeon, and, when required, a radiologist.
The heart team selected the THV type for each patient, and all those requiring a balloon-expandable (BE) THV were indicated for Myval Octacor, as it was the only BE THV available at our center. Consistent with standard practice in other TAVI centers, Myval was preferentially used in patients with large or extra-large annuli (tricuspid and bicuspid), low coronary heights, and horizontal aorta, while a self-expanding THV was used in patients with small annuli, small degenerated surgical bioprosthesis, or heavily calcified aortic annulus/aortic root. These criteria reflect our standard clinical decision making and ensures that each patient receives the THV type best suited to their anatomy. Limited valve availability contributed to the small sample size. Patients who underwent valve-in-valve procedures were excluded from the analysis.
All patients underwent preprocedural computed tomographic (CT) aortography, coronary angiography, Doppler examination of carotid arteries, and laboratory assessments. Additional diagnostic procedures or specialist examinations were conducted as deemed necessary.
Patients undergoing TAVI at our institution provided written informed consent for the use of anonymized clinical and procedural data for scientific and research purposes. The study was approved by the institutional ethics committee (No. 6902; December 30, 2024) and was conducted in accordance with the Declaration of Helsinki.
Device description
The Myval Octacor THV is a novel-generation, BE device featuring a cobalt-chromium frame composed of 2 rows of identical octagonal cells, designed to provide high radial strength and minimal foreshortening compared with other THVs. Its upper open-cell region maintains coronary access, while the lower closed-cell region enhances annular anchoring. Octacor incorporates an external polyethylene terephthalate (PET) sealing cuff that extends over half the frame height to reduce PVL. The Octacor series offers an extended size matrix, including intermediate and extra-large sizes (30.5 and 32 mm, respectively), allowing treatment of patients with extra-large annuli who were previously considered unsuitable for other THVs.16,18
The device’s Navigator Inception THV delivery system has an extremely flexible shaft and a deflectable tip, allowing for transit in tortuous and angulated vessels. Furthermore, Octacor is directly crimped over the balloon catheter before insertion into the interventional access, eliminating the need for in situ manipulation in the aorta. This feature reduces procedural time and complexity.
Procedural characteristics and post-procedural clinical outcomes
Outcomes were reported according to the Valve Academic Research Consortium-3 (VARC-3) definitions and were evaluated both immediately after the procedure and at the 30-day follow-up.19 The primary outcomes were all-cause mortality and cardiovascular death. Other outcomes included all stroke and disabling stroke, myocardial infarction, development of rhythm and conduction disturbances, new pacemaker implantation, vascular complications, life-threatening or disabling bleeding, renal failure, valve thrombosis, valve endocarditis, valve failure, and New York Heart Association (NYHA) functional class. Echocardiographic assessments included measurements of aortic valve area (AVA), peak and mean transvalvular gradients, and the degree of aortic regurgitation (AR).
Statistical analysis
Categorical variables were expressed as frequencies and percentages, and their analysis was performed using either Pearson’s χ2 test or Fisher’s exact test. For normally distributed variables, means with SDs were reported, while for non-normally distributed variables, medians with IQR were provided. Student’s t-test was employed to compare normally distributed continuous variables, whereas the Mann-Whitney U-test was used for non-normally distributed continuous variables. A double-sided P-value of 0.05 was considered statistically significant. All statistical analyses were conducted using SPSS Statistics, version 26 (IBM).
Results
Baseline characteristics
A total of 43 patients underwent TAVI using the Myval Octacor THV at our tertiary cardiac center in Belgrade, Serbia. The median age at the time of intervention was 81 (IQR: 7) years, 22 patients (51.2%) were female. Major cardiovascular risk factors were highly prevalent: arterial hypertension in all patients (100%), dyslipidaemia in 33 patients (76.74%), and diabetes mellitus in 12 patients (27.9%).
Among the 43 patients, 24 (55.8%) had documented coronary artery disease (CAD). Two patients (4.7%) had a history of coronary artery bypass grafting (CABG), 2 (4.7%) had both CABG and percutaneous coronary intervention (PCI), and 9 (20.9%) had undergone PCI. Notably, 4 of these 9 patients (9.3% of the total cohort) underwent PCI as part of the pre-TAVI evaluation and optimization process, while the remaining patients with CAD did not require further revascularization.
The mean left ventricular ejection fraction (LVEF) was 48.6 ± 11.5%, with a mean gradient of 55 mm Hg (IQR: 24) and maximal gradient of 89 mm Hg (IQR: 32). Table 1 presents the detailed demographic, clinical, and echocardiographic data at baseline. The mean STS-PROM score was 3.7%, with 28 patients (65%) classified as low risk, 12 patients (28%) as intermediate risk, and 3 patients (7%) as high risk. No patients were categorized as extreme surgical risk.
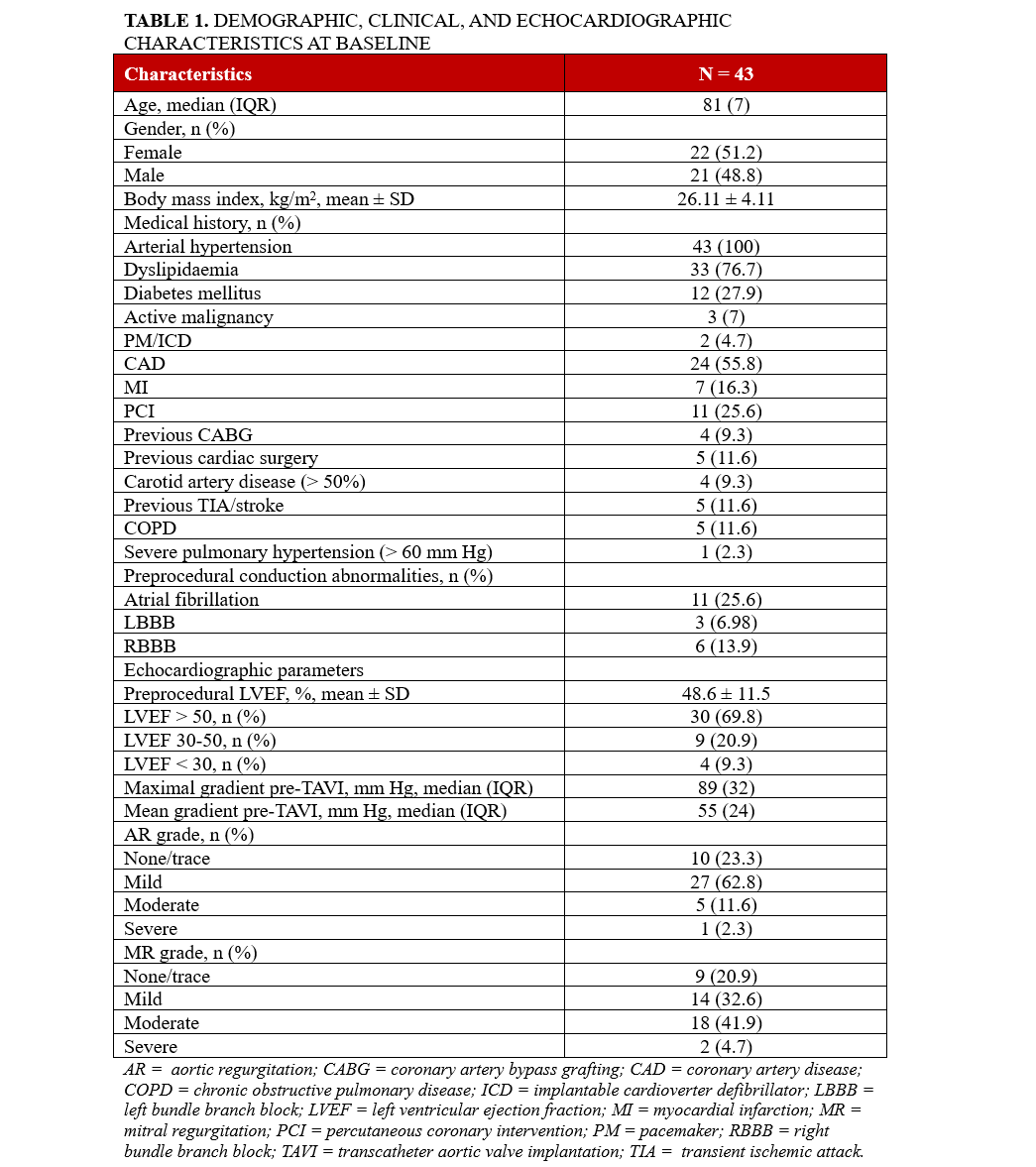
Aortic valve variables derived from CT
The perimeter-derived annulus diameter was 24.3 mm (IQR: 4.1), and the average annulus area was 454.1 mm2 (IQR: 133.9). The mean angle of the aortic LV was found to be 50.47 ± 10.29°. The mean diameter of the LV outflow tract was 24.1 mm (IQR: 4.1). Additional CT-derived variables are detailed in Table 2.
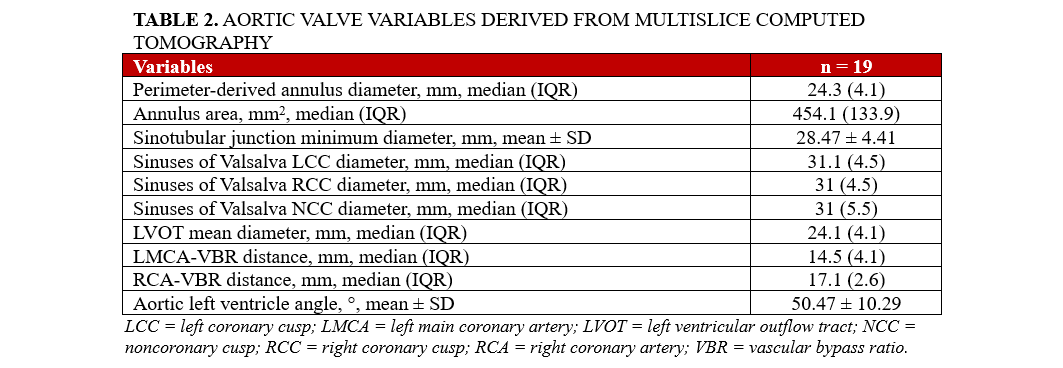
Procedural characteristics and post-procedural clinical outcomes
All patients underwent percutaneous access via the transfemoral route in local anaesthesia utilizing an expandable 14F Python introducer sheath (Meril Life Sciences). Cerebral protection devices were employed in 9 cases (20.9%). Predilatation was performed in 20 of 43 patients (46.5%), with 3 (15.0%) developing new-onset bundle branch block (BBB). Among the 23 patients without predilatation, 1 (4.3%) developed a new BBB. Subsequently, the BE Myval Octacor THV was successfully implanted under rapid pacing via a temporary pacemaker in the right ventricle in 23 cases (53.5%), and through left ventricular (LV) wire guidance in 20 cases (46.5%). Postdilatation was performed in 8 cases (18.6%); none had a new BBB. Postprocedural PVL assessment was carried out both echocardiographically and angiographically, and the mean post-TAVI pressure gradient was 4.5 mm Hg (IQR: 3), with a peak gradient of 7.5 mm Hg (IQR: 7). At discharge, most patients (28, 65.1%) were on single antiplatelet therapy (SAPT), with no patients receiving triple antithrombotic therapy (TAT). Pre- and postprocedural characteristics are shown in Table 3.
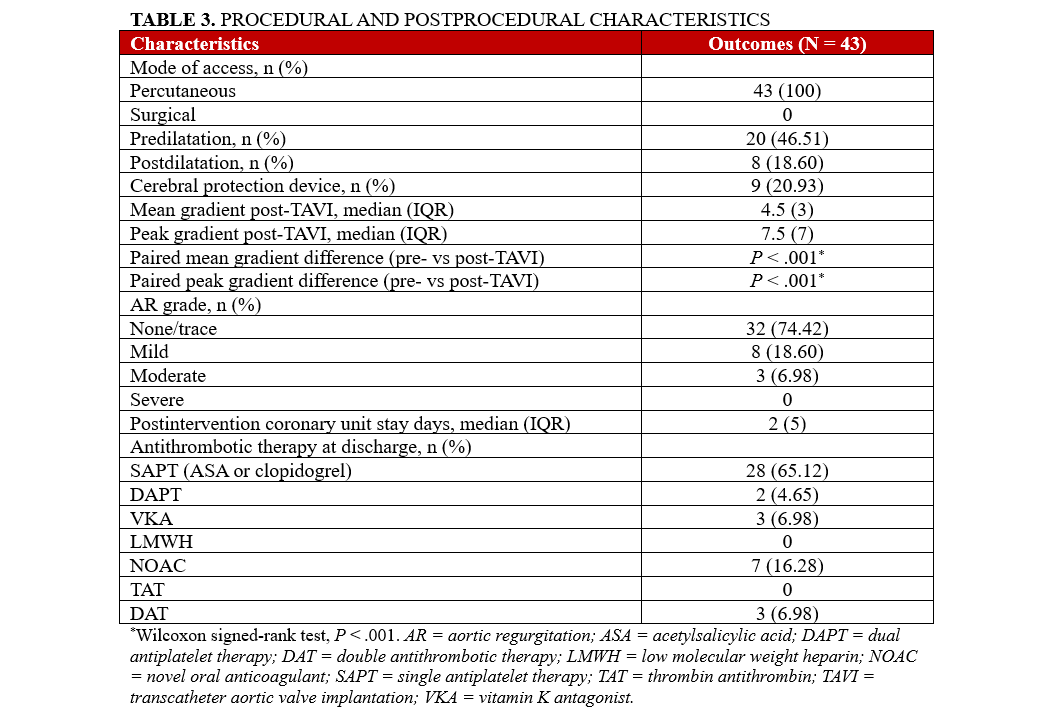
Post-procedure clinical outcomes
Results highlight favorable outcomes in individuals with severe AS who were implanted with the Myval Octacor THV. There were no incidences of death, acute kidney injury, stroke, severe AR, valve thrombosis, valve endocarditis, or valve failure post-procedure (Table 4). AR was absent or trace in the majority of patients (32, 74.4%). Mild AR was observed in 8 patients (18.6%), and moderate AR in 3 patients (7.0%). New cases of advanced atrioventricular block (AVB) or BBB were observed in 4 (9.3%) of patients. Furthermore, Type 2 (Bleeding Academic Research Consortium-3a) bleeding events occurred in 2 patients (4.7%), with no other complications reported during the index hospitalization.
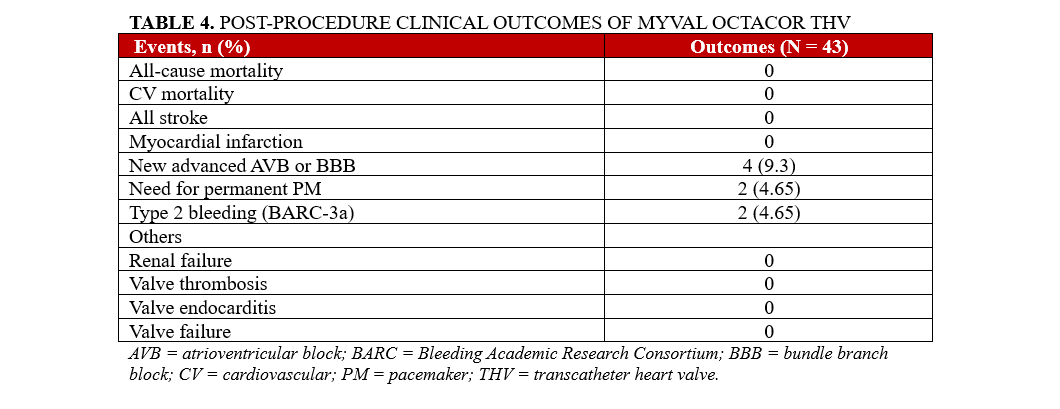
Two patients (4.7%) had a permanent pacemaker (PPM) prior to the procedure. Among the remaining 41 patients, new permanent pacemaker implantation (PPI) was required in 2 cases (4.9%) during the index hospitalization, both within the first 24 hours after TAVI. Additionally, 3 patients (7.3%) were readmitted within 7 days post-procedure because of conduction abnormalities detected through telemonitoring, necessitating PPI. In total, 5 of the 41 patients (12.2%) required new PPI at 30 days.
First follow-up (30 days post-TAVI)
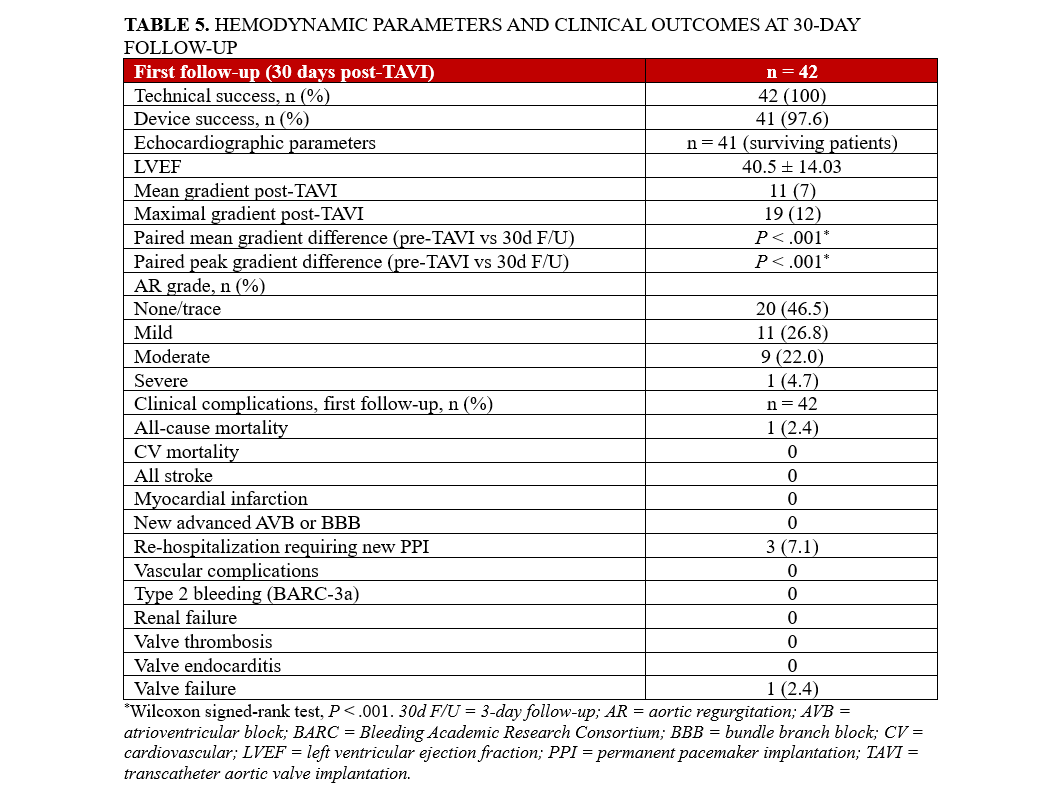
In 42 patients, follow‑up assessments were conducted post‑TAVI. One patient was lost and could not be contacted. Another patient died of pneumonia approximately 2 weeks after discharge from the index hospitalization. Echocardiographic measurements in the 41 surviving patients revealed a mean pressure gradient of 11 mm Hg (IQR: 7) and a peak gradient of 19 mm Hg (IQR: 12). The mean preprocedural LVEF was 48.6 ± 11.5%. At 30-day follow-up, the mean LVEF was 49.7 ± 12.4%, demonstrating no significant change in LV systolic function. These results suggest that the TAVI procedure preserved cardiac function in this patient cohort. AR was mild in 11 patients (26.8 %) and moderate in 9 (22.0 %). One patient experienced valve failure, presenting with significant PVL, and underwent balloon dilatation of the implanted TAVI valve. The remainder exhibited no regurgitation. Additionally, 3 (7.3 %) were readmitted within 7 days because of conduction abnormalities detected via telemonitoring, requiring PPI. At follow-up, 29 of the 41 patients (70.7 %) showed symptomatic improvement in NYHA Class by I or II levels, 10 (24.4 %) remained stable in NYHA Class II, and 2 (4.9 %) remained in NYHA Class III. No cardiovascular death, acute kidney injury, stroke, valve thrombosis, or valve endocarditis occurred. Detailed outcomes are presented in Table 5.
Discussion
This observational study documents the early outcomes observed during the first 30 days post-treatment in 43 patients diagnosed with severe AS who underwent TAVI with the Myval Octacor THV. The technical success rate at 30 days was 100%, device success was 97.6%, and there were no cardiovascular deaths observed. Significant improvement was observed in hemodynamic parameters through 30-day post-treatment. The mean pressure gradient declined significantly from 55 mm Hg (IQR: 24) at baseline to 11 mm Hg (IQR: 7) post‑30 days (P < .001), and the peak pressure gradient dropped from 89 mm Hg (IQR: 32) to 19 mm Hg (IQR: 12), also highly significant (P < .001).
Several real-world studies have reported favorable outcomes with the Myval THV. In the OCTACOR India study (n = 123), the peak pressure gradient significantly decreased from 88.01 ± 25.28 mm Hg at baseline to 18.85 ± 7.66 mm Hg at 30 days, with a 30-day all-cause mortality rate of 1.6%, 98.4% device success, and 100% technical success.16 Similarly, the MATCH-BALL study (n = 130), reported a procedural success rate of 93.2%, a 30-day mortality rate of 0.97%, and a new PPI rate of 5.8%.20 In another real-world study of 68 patients with severe AS, Elkoumy et al reported 93% device success, a 30-day mortality rate of 3%, and a PPI rate of 8.5%.21 These outcomes support the safety and efficacy of Myval THV across diverse patient populations.
Previous studies evaluating earlier versions of the Myval THV have demonstrated consistent clinical safety and procedural efficiency across various populations. A retrospective TAVI-SAVR comparison (n = 166; Myval group: n = 58) reported low rates of PVL, with moderate or greater PVL in only 6.9% of TAVI patients.22 A 2-year follow-up of Myval THV showed sustained technical success and no structural valve deterioration, highlighting the durability of the valve design.23 In Serbia, Boljevic et al examined the initial experience with the BE Myval THV in 13 patients and reported excellent safety outcomes, including no clinically significant AR, PVL, or major complications.24 These findings support the favorable safety profile of the Myval platform and are in line with the performance observed in our current evaluation of the newer-generation Myval Octacor THV.
The use of a 14F Python introducer sheath facilitated the percutaneous transfemoral access, which plays a critical factor in minimizing vascular complications. Previous studies with earlier versions of the Myval THV have documented the utilization of sheaths smaller than or equal to 19F.25,26 A retrospective analysis of the impact of sheaths on TAVI outcomes specified that sheaths with lower profiles are linked to a decreased rate of major vascular complications (0.5% vs 10.5%; P < .001) and major bleeding (3.4% vs 8.3%; P = .038) compared with high-profile sheaths.27 Stortecky et al reported that low-profile F sheaths (< 19F) were associated with significantly lower major vascular complications compared with high-profile (> 22F) delivery sheaths (6.4% vs 25%; P < .0001).28 Studies that investigated a large diameter delivery system, such as the PARTNER 1A, PARTNER 1B, and the SOURCE registry, have documented major vascular complication rates of 13.7%, 17.5%, and 17.9%, respectively.29 Our findings revealed no vascular complications during index hospitalization and at 30 days post-treatment. This indicates that low-profile sheaths may have better manoeuvring capabilities.
Predilatation is a major predisposing factor for valve oversizing and high prosthesis-induced stretching and annular trauma leading to new-onset left bundle branch block (LBBB) and high-grade AVB.28 The occurrence of these conduction abnormalities often leads to the requirement of a permanent pacemaker, a major complication associated with the procedure. Atrial fibrillation (AF) was reported prior to intervention in 11 patients, LBBB in 3 patients, and right bundle branch block (RBBB) in 6 patients. New cases of advanced AVB or BBB were observed in 4 patients. PPI was required in 5 patients (11.6%), potentially because of a combination of pre-existing conduction abnormalities, new-onset BBB, predilatation, and other procedural or patient-specific factors. In our study, predilatation was necessitated in almost half of the population (46.5%) to facilitate prosthesis delivery and optimal expansion to enhance hemodynamic stability during deployment.29
In the OCTACOR India study, the PPI requirement rate was 10.6% during index hospitalization. Baseline analysis indicated AF (8.1%), LBBB (6.5%), and RBBB (2.4%).16 According to a multicenter study conducted on the Evolut FX system for TAVI, 10.2% of patients had RBBB at baseline and 11.9% of patients required PPI within 30 days post-treatment.30 In the EVAL Registry 6-month follow-up study (n = 166; Myval group n = 58), the rate of PPI was 11%; moderate annulus calcification was 16%, and baseline AF was 31%.22 The ACURATE-neo valve study and the Portico with FlexNav system (Abbott) study reported PPI rates of 10% and 14.6%, respectively.30 All these studies reflect the close association with pre-existing conduction abnormalities and the requirement for PPI. The presence of RBBB at baseline has been reported as a predisposing factor of PPI, with an increased risk up to 47 times.31 As previously stated, predilatation can have a significant impact, alongside conduction abnormalities, on the necessity for PPI. Although there has been a notable reduction in the occurrence of PPI and conduction abnormalities after the advent of new-generation TAVI modalities, a pressing concern over these adverse outcomes persists.32
Our 30-day post-treatment reported no stroke incidents, renal failure, or valve thrombosis, validating the evidence of early safety of the device. Eggebrecht et al reported that strokes or transient ischemic attacks were associated with TAVI at the rate of 3.3 ± 1.8%. Many of these strokes were classified as major strokes, causing a 3.5-fold increase in mortality and death within 30 days of treatment.33,34 The OCTACOR India study also reported no stroke incidents, confirming the procedural and device success of Myval Octacor THV.16 Major bleeding was also not reported in any patient during the 30-day follow-up period. Valve failure was reported in 1 patient, possibly due to significant AR, which was revealed through echocardiographic assessment. This finding is significant because PVL is a well-recognized complication of TAVI, often leading to adverse outcomes if severe.
Comparative evaluation of the Myval THV with other established transcatheter valves is essential as its use expands globally. The LANDMARK trial, a large, multicenter, randomized study involving 768 patients across 31 hospitals in 16 countries compared the newer-generation Myval Octacor THV with contemporary valves such as Sapien and Evolut in patients with severe symptomatic native AS. Early results demonstrated noninferiority of the Myval Octacor THV (25%) compared with other valves (27%), with a risk difference of -2.3% (upper 95% CI, 3.8; P < .0001 for noninferiority. Our findings also complement those of the OCTACOR-EU study by providing additional insight into early outcomes in a larger, dedicated, single-center cohort. This experience further supports the safety and performance of the Myval Octacor THV in routine clinical practice. Together, these findings reinforce Myval’s as a competitive alternative in the current TAVI landscape, and forthcoming long-term data from this trial will provide further insight into its durability and clinical outcomes.17
Limitations
Our study has several limitations. First, the low sample size of 43 patients limits the generalizability of findings. Studies conducted in multiple centers with large sample sizes and across broader populations are needed to confirm our findings. Second, this is a retrospective, observational study; therefore, these results are subject to selection bias and no causality can be derived. Third, the short-term follow-up of 30 days may misconstrue both long-term durability and potential valve-related adverse events of the Myval Octacor THV. Longer follow-up is necessary to ensure the durability of the valve. Fourth, this study was conducted in a single Serbian center; therefore, demographic differences and patient characteristics may limit the generalizability of our results. We performed extensive pre- and post-procedure assessments in this study; however, several procedural variables and operator-dependent factors were not assessed, and their omission may limit the reproducibility of our results. To address these limitations, studies such as the LANDMARK trial (NCT04275726) and the COMPARE-TAVI trial (NCT04443023) with larger samples, multicenter designs, and longer follow-up periods are underway to provide more comprehensive and generalizable evidence.
Conclusions
This retrospective study of 43 patients undergoing TAVI with the Myval Octacor THV showed 100% technical success, a significant reduction in mean pressure gradient, and no in-hospital mortality. At 30 days, there were no cardiovascular deaths, strokes, or major vascular complications; 1 non-cardiovascular death occurred, 1 valve failure was successfully treated, the pacemaker implantation rate was 7.3%, and most patients showed clinical and functional improvement. These early outcomes highlight the clinical safety, procedural effectiveness, and hemodynamic benefits of the Myval Octacor THV, particularly in patients requiring a BE system with a low-profile delivery. Larger, multicenter studies with long-term follow-up are needed to confirm these findings and further assess valve durability and clinical impact over time.
Affiliations and Disclosures
Valentina Balint, MD1; Mihajlo Farkic, MD1; Jovan Petrovic, MD1; Matija Furtula, MD1; Milos D. Babic, MD1; Dragan Topic, MD1; Ida Subotic, MD1; Vladimir Zobenica, MD1; Alfonso Ielasi, MD2; Luca Testa, MD, PhD3; Milovan Bojic, MD, PhD1,4; Aleksandra Nikolic, MD, PhD1,5
From the 1Institute for Cardiovascular Diseases Dedinje, Belgrade, Serbia; 2U.O. Cardiologia Ospedaliera, IRCCS Ospedale Galeazzi-Sant’Ambrogio, Milan, Italy; 3Department of Cardiology, IRCCS Policlinico San Donato, San Donato Milanese, Milan, Italy; 4Faculty of Medicine, University of Banja Luka, Banja Luka, Bosnia and Herzegovina; 5Faculty of Medicine, University of Belgrade, Belgrade, Serbia.
Disclosures: The authors report no financial relationships or conflicts of interest regarding the content herein.
Address for correspondence: Valentina Balint, MD, Institute for Cardiovascular Diseases Dedinje, Heroja Milana Tepica 1, Belgrade, Serbia. Email: tina.balint@gmail.com
References
- Aluru JS, Barsouk A, Saginala K, Rawla P, Barsouk A. Valvular heart disease epidemiology. Med Sci (Basel). 2022;10(2):32. doi:10.3390/medsci10020032
- Vahanian A, Beyersdorf F, Praz F, et al; ESC/EACTS Scientific Document Group. 2021 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J. 2022;43(7):561-632. doi:10.1093/eurheartj/ehab395
- Otto CM, Nishimura RA, Bonow RO, et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2021;143(5):e72-e227. doi:10.1161/CIR.0000000000000923
- Deeb GM, Reardon MJ, Chetcuti S, et al; CoreValve US Clinical Investigators. 3-year outcomes in high-risk patients who underwent surgical or transcatheter aortic valve replacement. J Am Coll Cardiol. 2016;67(22):2565-2574. doi:10.1016/j.jacc.2016.03.506
- Smith CR, Leon MB, Mack MJ, et al; PARTNER Trial Investigators. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med. 2011;364(23):2187-2198. doi:10.1056/NEJMoa1103510
- Mack MJ, Leon MB, Smith CR, et al; PARTNER 1 trial investigators. 5-year outcomes of transcatheter aortic valve replacement or surgical aortic valve replacement for high surgical risk patients with aortic stenosis (PARTNER 1): a randomised controlled trial. Lancet. 2015;385(9986):2477-2484. doi:10.1016/S0140-6736(15)60308-7
- Leon MB, Smith CR, Mack MJ, et al; PARTNER 2 Investigators. Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N Engl J Med. 2016;374(17):1609-1620. doi:10.1056/NEJMoa1514616
- Thourani VH, Kodali S, Makkar RR, et al. Transcatheter aortic valve replacement versus surgical valve replacement in intermediate-risk patients: a propensity score analysis. Lancet. 2016;387(10034):2218-2225. doi:10.1016/S0140-6736(16)30073-3
- Reardon MJ, Van Mieghem NM, Popma JJ, et al; SURTAVI Investigators. Surgical or transcatheter aortic-valve replacement in intermediate-risk patients. N Engl J Med. 2017;376(14):1321-1331. doi:10.1056/NEJMoa1700456
- Makkar RR, Thourani VH, Mack MJ, et al; PARTNER 2 Investigators. Five-year outcomes of transcatheter or surgical aortic-valve replacement. N Engl J Med. 2020;382(9):799-809. doi:10.1056/NEJMoa1910555
- Mack MJ, Leon MB, Thourani VH, et al; PARTNER 3 Investigators. Transcatheter aortic-valve replacement with a balloon-expandable valve in low-risk patients. N Engl J Med. 2019;380(18):1695-1705. doi:10.1056/NEJMoa1814052
- Diegoli H, Alves MRD, Okumura LM, Kroll C, Silveira D, Furlan LHP. Transcatheter valve replacement in patients with aortic valve stenosis: an overview of systematic reviews and meta-analysis with different populations. Arq Bras Cardiol. 2023;120(7):e20220701. doi:10.36660/abc.20220701
- Généreux P, Head SJ, Van Mieghem NM, et al. Clinical outcomes after transcatheter aortic valve replacement using valve academic research consortium definitions: a weighted meta-analysis of 3,519 patients from 16 studies. J Am Coll Cardiol. 2012;59(25):2317-2326. doi:10.1016/j.jacc.2012.02.022
- Nazif TM, Chen S, George I, et al. New-onset left bundle branch block after transcatheter aortic valve replacement is associated with adverse long-term clinical outcomes in intermediate-risk patients: an analysis from the PARTNER II trial. Eur Heart J. 2019;40(27):2218-2227. doi:10.1093/eurheartj/ehz227
- Auffret V, Puri R, Urena M, et al. Conduction disturbances after transcatheter aortic valve replacement: current status and future perspectives. Circulation. 2017;136(11):1049-1069. doi:10.1161/CIRCULATIONAHA.117.028352
- Jose J, Mandalay A, Cholenahally MN, et al. Safety and effectiveness of the novel Myval Octacor transcatheter heart valve in severe, symptomatic aortic valve stenosis - a real-world Indian experience (The OCTACOR India Study). Cardiovasc Revasc Med. 2024;63:1-7. doi:10.1016/j.carrev.2024.01.016
- Ielasi A, Caminiti R, Giordano A, et al. Immediate and early outcomes following myval octacor transcatheter heart valve implantation for the treatment of patients with severe aortic valve stenosis: the OCTACOR-EU study. Catheter Cardiovasc Interv. 2025;106(1):511-520. doi:10.1002/ccd.31563
- Holzamer A, Bedogni F, van Wyk P, et al. Performance of the 32 mm Myval transcatheter heart valve for treatment of aortic stenosis in patients with extremely large aortic annuli in real-world scenario: first global, multicenter experience. Catheter Cardiovasc Interv. 2023;102(7):1364-1375. doi:10.1002/ccd.30820.
- VARC-3 WRITING COMMITTEE; Généreux P, Piazza N, et al. Valve Academic Research Consortium 3: updated endpoint definitions for aortic valve clinical research. Eur Heart J. 2021;42(19):1825-1857. doi:10.1093/eurheartj/ehaa799
- Delgado-Arana JR, Gordillo-Monge MX, Halim J, et al. Early clinical and haemodynamic matched comparison of balloon-expandable valves. Heart. 2022;108(9):725-732. doi:10.1136/heartjnl-2021-319349
- Elkoumy A, Jose J, Gunasekaran S, et al. Angiographic quantification of aortic regurgitation following myval octacor implantation; independent core lab adjudication. Int J Cardiol. 2023;382:68-75. doi:10.1016/j.ijcard.2023.04.003
- Barki M, Ielasi A, Buono A, et al. Clinical comparison of a novel balloon-expandable versus a self-expanding transcatheter heart valve for the treatment of patients with severe aortic valve stenosis: the EVAL registry. J Clin Med. 2022;11(4):959. doi:10.3390/jcm11040959
- Testa L, Criscione E, Popolo Rubbio A, et al. Safety and performance parameters of the Myval transcatheter aortic valve bioprosthesis: the SAPPHIRE prospective registry. Cardiovasc Revasc Med. 2023;55:22-27. doi:10.1016/j.carrev.2023.04.014
- Boljevic D, Bojic M, Farkic M, et al. Early outcomes of a next-generation balloon-expandable transcatheter heart valve-the Myval system: a single-center experience from Serbia. J Cardiol Cardiovasc Med. 2023;8(2):072-080. doi:10.29328/journal.jccm.1001156
- Elkoumy A, Jose J, Terkelsen CJ, et al. Safety and efficacy of Myval implantation in patients with severe bicuspid aortic valve stenosis-a multicenter real-world experience. J Clin Med. 2022;11(2):443. doi:10.3390/jcm11020443
- Ramesh RN, Daggubati R, Babu PR. Transcatheter aortic valve implantation in two high-risk patients with low coronary ostial heights using the novel balloon-expandable Myval valve. J Cardiol Cardiovasc Med. 2023;8(2):089-099. doi:10.29328/journal.jccm.1001159
- Barbanti M, Binder RK, Freeman M, Wood DA, Leipsic J, Cheung A, et al. Impact of low-profile sheaths on vascular complications during transfemoral transcatheter aortic valve replacement. EuroIntervention 2013; 9(8): 929-35.
- Halapas A, Koliastasis L, Doundoulakis I, Antoniou CK, Stefanadis C, Tsiachris D. Transcatheter aortic valve implantation and conduction disturbances: focus on clinical implications. J Cardiovasc Dev Dis. 2023;10(11):469. doi:10.3390/jcdd10110469
- Pagnesi M, Baldetti L, Del Sole P, et al. Predilatation prior to transcatheter aortic valve implantation: is it still a prerequisite? Interv Cardiol. 2017;12(2):116-125. doi:10.15420/icr.2017:17:2
- Zaid S, Attizzani GF, Krishnamoorthy P, et al. First-in-human multicenter experience of the newest generation supra-annular self-expanding Evolut FX TAVR system. JACC Cardiovasc Interv. 2023;16(13):1626-1635. doi:10.1016/j.jcin.2023.05.004
- Siontis GC, Jüni P, Pilgrim T, et al. Predictors of permanent pacemaker implantation in patients with severe aortic stenosis undergoing TAVR: a meta-analysis. J Am Coll Cardiol. 2014;64(2):129-140. doi:10.1016/j.jacc.2014.04.033
- Kalogeropoulos AS, Redwood SR, Allen CJ, et al. A 20-year journey in transcatheter aortic valve implantation: evolution to current eminence. Front Cardiovasc Med. 2022;9:971762. doi:10.3389/fcvm.2022.971762
- Eggebrecht H, Schmermund A, Voigtländer T, Kahlert P, Erbel R, Mehta RH. Risk of stroke after transcatheter aortic valve implantation (TAVI): a meta-analysis of 10,037 published patients. EuroIntervention. 2012;8(1):129-138. doi:10.4244/EIJV8I1A20
- Werner N, Zeymer U, Schneider S, et al; German Transcatheter Aortic Valve Interventions-Registry Investigators. Incidence and clinical impact of stroke complicating transcatheter aortic valve implantation: results from the German TAVI registry. Catheter Cardiovasc Interv. 2016;88(4):644-653. doi:10.1002/ccd.26612