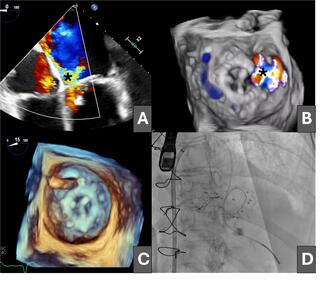
Long-Term Outcomes After Transcatheter Tricuspid Valve-in-Valve Replacement
An Interview With Josep Rodés-Cabau, MD, PhD
An Interview With Josep Rodés-Cabau, MD, PhD
Key Summary
-
Long-term follow-up of this single-center study supports the overall safety and feasibility of tricuspid ViV procedures in patients with failed surgical bioprosthetic valves, including younger and congenital populations.
-
Valve degeneration can occur over time, underscoring the importance of ongoing surveillance and individualized planning for potential repeat interventions, particularly in relation to postprocedural valve gradients.
-
Anticoagulation for a limited period after ViV may be reasonable when tolerated, and pacemaker lead jailing should be avoided due to potential pacing complications, highlighting the need for multidisciplinary care and follow-up.
Dr Rodés-Cabau shares background and insights on his article, "Long-Term Outcomes After Transcatheter Tricuspid Valve-in-Valve Replacement."
Hello, I am Josep Rodés-Cabau, interventional cardiologist at the Quebec Heart and Lung Institute. It is a pleasure to be here today to provide some highlights about our article that has just been published in the Journal of Invasive Cardiology about the long-term follow-up of patients undergoing tricuspid valve-in-valve procedures.
Based on your 6-year mean follow-up, what hemodynamic or clinical markers would you consider most predictive of long-term valve durability in VIV patients?
Unfortunately, the number of patients included in the study was very limited, and this precluded us from determining any reliable clinical or hemodynamic markers predicting bioprosthetic valve failure in this cohort. For that purpose, we will need much larger studies.
Your cohort included a relatively young population, mean age of 45. How should cardiologists think about the role of tricuspid VIV in younger patients who may require further interventions, and what does your data suggest about timing?
This is an important question. The reason for the younger age is because about half of the patients were congenital patients. This is very frequent in the right side and in surgical tricuspid valve replacement, so this is not surprising. I would say that we now have very solid data about the safety of these procedures. Procedural success in our series was 100%, and this is the case, or close to, in most published series.
So, despite the younger age, we have to consider that these patients have already had at least one surgical intervention, and it would be reasonable, when these surgical valves fail, to offer them a transcatheter valve-in-valve procedure.
When you look at the mid- to long-term follow-up, you can expect that many of these valves will fail with time. In many cases, particularly in those where your residual gradient is relatively low, you could anticipate the possibility of another valve-in-valve procedure; this is a place where you don't have coronary arteries, like in TAVI procedures, etc, meaning that the patient could receive more than one valve-in-valve procedure. However, there are some patients, such as those with more elevated gradients, where this may be a suboptimal option.
Antithrombotic strategies varied widely in your study. Can you make any recommendations on the optimal regimen based on the current results, or do you have any future research planned to assess antithrombotic management in this population?
Another very important question. It's true—this was a real-world, single-center registry, and in fact, half of the patients left the hospital with anticoagulation therapy and the other half had antiplatelet therapy, but our tendency is to give some anticoagulation to all these patients, at least for a few months. We had at least one patient where we diagnosed increasing transvalvular gradients, probably related to subclinical thrombosis. Following the recommendations in surgical bioprosthesis, I think that the following tricuspid valve-in-valve procedures, anticoagulation at least for a few months is probably the best strategy if the patient can tolerate anticoagulation therapy.
Our plan is to have a much larger number of patients in a multicenter study, and this will allow us to determine what is probably the most appropriate antithrombotic strategy. In the end, maybe we will need a randomized trial—the problem is that the number of valve-in-valve tricuspid procedures is not very high and it might be difficult to have a properly designed randomized trial in this space.
Beyond antithrombotic management, did your findings highlight any additional questions or avenues for further research that warrant further investigation?
Yes, I would say that one aspect to highlight is the importance of a continued follow-up of these patients. As you have seen in the study, we had a rate of valve degeneration of 17% at 6 years%. This is a bit higherthan the valve failure rate that we see in, for example, aortic valves. And it's not really a big surprise, because we also know that surgical valves in this position may tend to degenerate more rapidly, meaning that I think that we need this continued follow-up.
Another aspect that was highlighted by the study is that some of the patients included in this series had prior pacemakers. There were 3 patients with pacemaker leads that were jailed between the transcatheter valve and the surgical valve, and in two of these patients, there were increasing pacing thresholds with the EP controls. And in fact, in one of them, we required a new transvenous lead and battery replacement.
We have just published a consensus statement, including electrophysiologists, interventional cardiologists, and cardiac surgeons, recommending not to jail the pacemaker leads in these patients. If there is, for example, an endocarditis issue, this may be a big issue, preventing lead extraction in these cases. But we can also have this type of issue (increasing pacing thresholds) related to pacemaker lead jailing, as demonstrated in our study and other studies. I think this is an important lesson, and we will definitely need more data on these jail leads in order to provide more definite recommendations. But our study provided a word of caution regarding the management of these patients.
Though the sample size was small, as you mentioned, are you able to make any determinations about patient selection criteria that may help clinicians identify those most likely to benefit from tricuspid ViV intervention?
I would say that in our experience, the patients that may benefit the most are those presenting with severe or massive tricuspid regurgitation. In these patients, most times, you end with relatively low gradients and no residual leak. This being said, I think that the procedure is so safe that it should be considered irrespective of the type of surgical bioprosthetic failure. It is a procedure that can be done without general anesthesia, and it does not require hospitalization; nowadays, we are discharging these patients the same day. Overall, it's a procedure where the patient can benefit without a lot of risk. I think that all cardiologists who follow patients with surgical tricuspid valves should consider transcatheter tricuspid valve-in-valve as a valid alternative in case of bioprosthetic valve failure.
However, with the limited number of patients included in our study, we cannot provide any specific recommendation regarding which patients could benefit more than others. I think that we will need larger series of patients. This is a work in progress, with a multicenter international registry, trying to collect data on long-term outcomes in this specific group of patients.
Our study was the first providing very long-term data on these patients, and I'm sure that this will stimulate further studies in this important field.
© 2025 HMP Global. All Rights Reserved.
Any views and opinions expressed are those of the author(s) and/or participants and do not necessarily reflect the views, policy, or position of the Journal of Invasive Cardiology or HMP Global, their employees, and affiliates.