Off‑Label Use of a Ventricular Septal Defect Occluder for Large Mitral Paravalvular Leak in the Early Postoperative Period: A Case Report
© 2026 HMP Global. All Rights Reserved.
Any views and opinions expressed are those of the author(s) and/or participants and do not necessarily reflect the views, policy, or position of the Journal of Invasive Cardiology or HMP Global, their employees, and affiliates.
J INVASIVE CARDIOL 2026. doi:10.25270/jic/25.00347. Epub January 6, 2026.
Percutaneous closure of paravalvular leaks (PVL) in the immediate postoperative period is not recommended because of the fragility of the tissues. These defects can occasionally be large, leading to hemodynamic compromise of the patient and representing a challenge for the intervention.
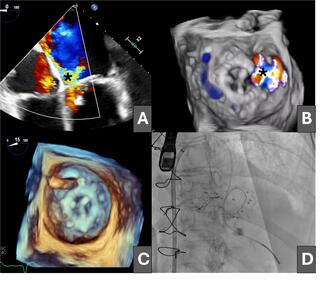
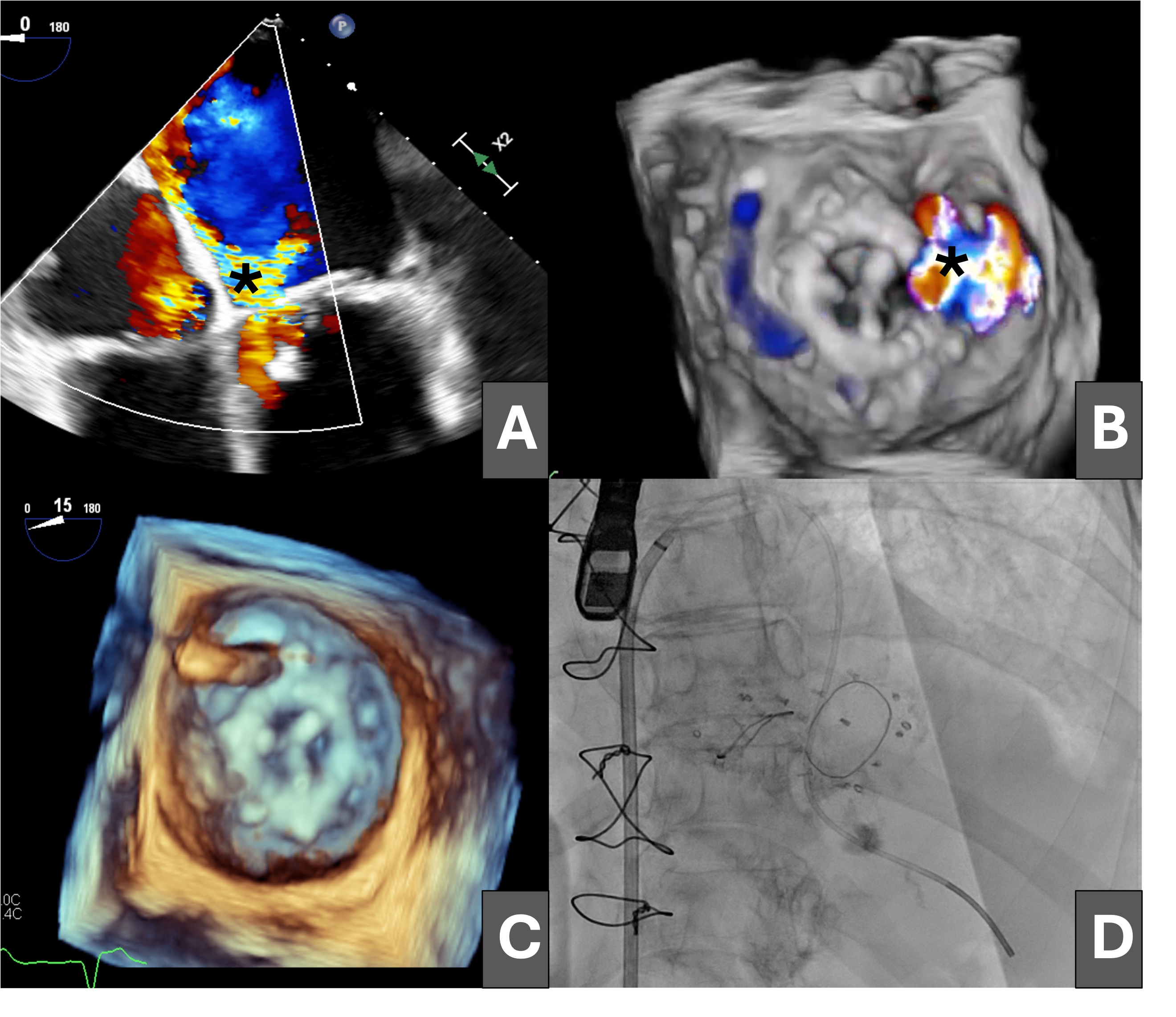
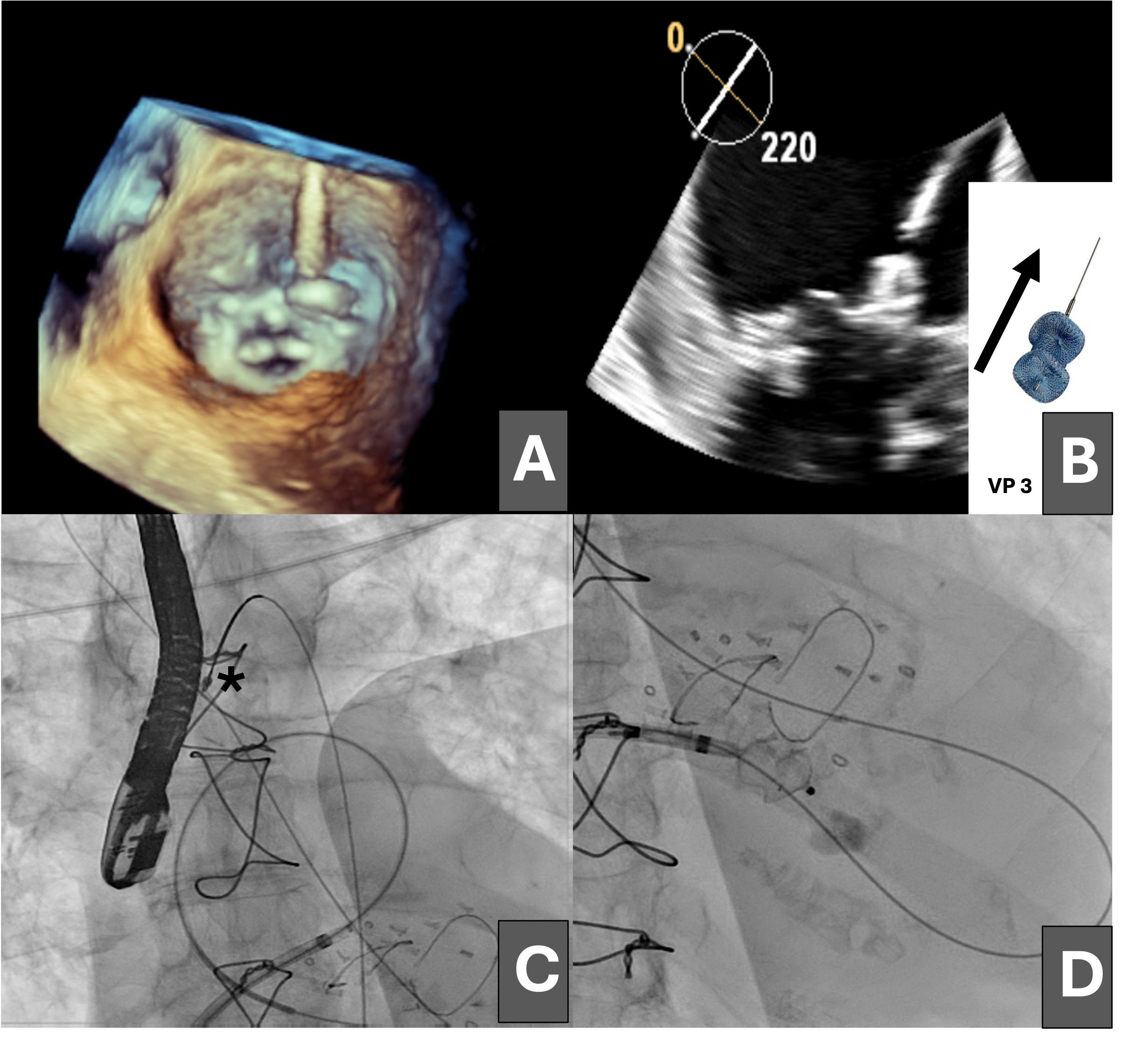
An 81-year-old woman was admitted for heart failure in the context of degenerative valvular disease; she had previously undergone mitral and aortic valve replacement with bioprostheses and tricuspid annuloplasty. The postoperative course was complicated by progressive heart failure and cardiogenic shock secondary to severe mitral regurgitation due to a large anterolateral mitral PVL (Figure 1A and B [asterisk]; Videos 1 and 2).
Having been ruled out for repeat surgery, percutaneous closure of the leak was performed via an initial antegrade approach, crossing the defect from the left atrium with a hydrophilic guidewire (Figure 1C), which was exchanged for a high-support guidewire through a multipurpose catheter advanced into the left ventricle (Figure 1D).
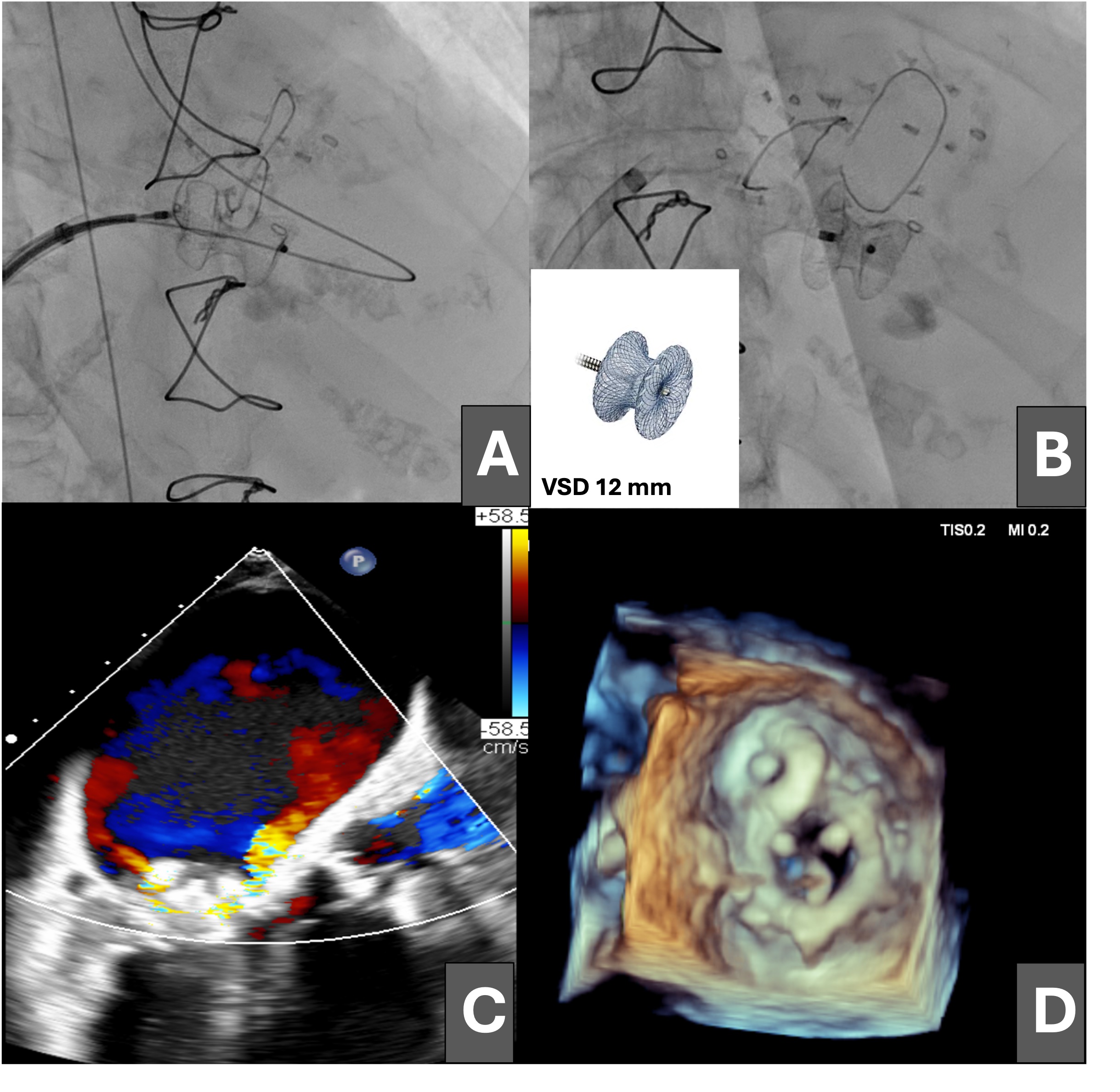
Subsequently, a 14 × 5-mm Amplatzer Vascular Plug III (VP3, Abbott) was advanced through the defect, but it prolapsed into the left atrium (Figure 2A and B). The dehiscence was crossed again, establishing an arteriovenous loop by snaring the guidewire in the ascending aorta (Figure 2C) and maintaining the venoarterial circuit; however, stability of the VP3 device could not be achieved because of the large size of the leak (Figure 2D).
Finally, a 12-mm ventricular septal defect (VSD) closure device was advanced (Figure 3A and B) and released with adequate compression, resulting in moderate residual mitral regurgitation as shown on transesophageal echocardiography (Figure 3C and D; Videos 3 and 4).
In select high‑risk patients with large, early postoperative mitral PVL not amenable to dedicated PVL devices, the off‑label use of a VSD occluder may provide a feasible alternative. Careful imaging, valve‑prosthesis compatibility assessment, and multidisciplinary decision making are paramount.
Affiliations and Disclosures
Leire Unzué, MD, PhD1; Lorena Martín-Polo, PhD, MD2; Ángel González-Pinto, PhD, MD3
From the Units of 1Interventional Cardiology, 2Cardiac Image, and 3Cardiac Surgery, Centro Integral de Enfermedades Cardiovasculares, Hospital Universitario HM Montepríncipe, HM Hospitales, Madrid, Spain.
Disclosures: The authors report no financial relationships or conflicts of interest regarding the content herein.
Consent statement: The authors confirm that written consent for the submission and publication of this case, including images, has been obtained from the patient in line with COPE guidance.
Address for correspondence: Leire Unzué, MD, PhD, Hospital Universitario HM Montepríncipe, Avda Montepríncipe 25, 28660 Boadilla del Monte, Madrid, Spain. Email: leireunzue@yahoo.es; X: @LUnzue