SWITCHing to Distal RaiLtracking Technique for Rotational Atherectomy Procedures: Short-Term Outcomes and Impact on Clinical Practice (The SWITCH D-RIL Registry)
© 2026 HMP Global. All Rights Reserved.
Any views and opinions expressed are those of the author(s) and/or participants and do not necessarily reflect the views, policy, or position of the Journal of Invasive Cardiology or HMP Global, their employees, and affiliates.
J INVASIVE CARDIOL 2026. doi:10.25270/jic/25.00355. Epub January 26, 2026.
Abstract
Objectives. Despite the preference for transradial access (TRA) in percutaneous coronary interventions (PCI), many operators still use large-bore guiding catheters through the transfemoral approach (TFA), especially in complex cases. This study evaluated the feasibility, safety, and efficacy of the Distal RailTracking (DRT) technique, a sheathless approach via distal radial access (DRA), and investigated its impact on clinical practice.
Methods. The multicenter SWITCH D-RIL study enrolled patients who required treatment of severely calcified coronary disease by rotational atherectomy, and compared those who underwent PCI before (pre-DRT, n = 97) and after (post-DRT, n = 99) DRT was adopted as the primary approach.
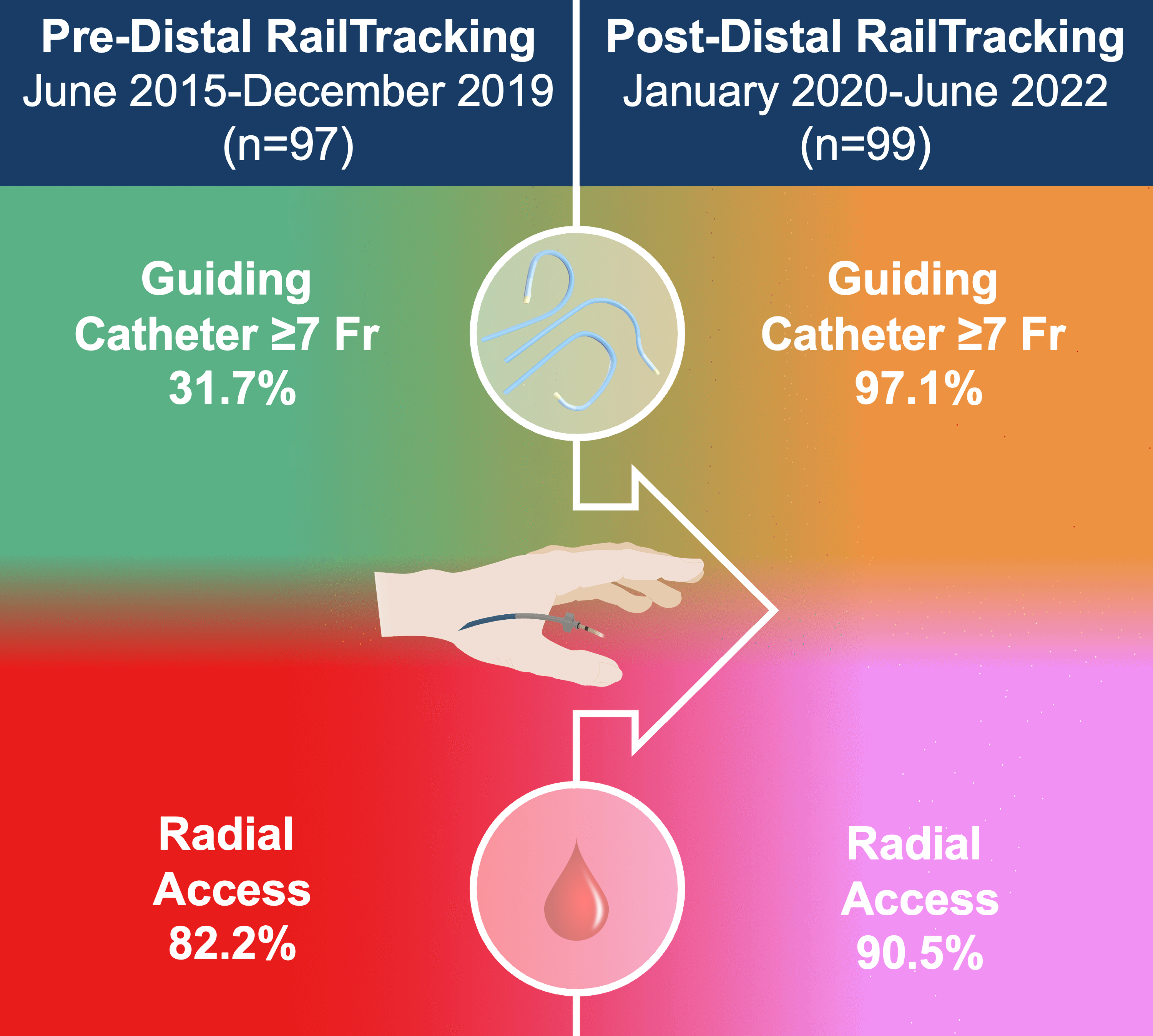
Results. Procedural success rates without access site crossover were similar between groups. No significant differences were found in periprocedural complications, in-hospital and 30-day major adverse cardiovascular events, and access- and non-access-related major bleedings. The post-DRT group exhibited a significantly higher use of large-bore guiding catheters (97.1% vs 31.7%). Overall, DRA was used in 90.5% of cases in the post-DRT group, with TFA accounting for 9.5%; the pre-DRT group primarily employed conventional TRA (82.2%), with the remaining cases involving TFA and 1 transbrachial access. The post-DRT group demonstrated increased usage of burrs larger than 1.5 mm (51.4% vs 13.9%) and additional calcium modifying tools (42.9% vs 24.8%).
Conclusions. The DRT technique demonstrated feasibility, safety, and efficacy in treating severely calcified coronary disease. This study highlights the reliability of DRA, even when large bore guiding catheters are necessary, emphasizing its potential to provide a safer approach while ensuring radial artery preservation.
Introduction
Both European and American guidelines strongly recommend transradial access (TRA) as the highest standard of care for percutaneous coronary intervention (PCI) because of its numerous advantages over transfemoral access (TFA) in both elective and acute settings.1-3 However, complex percutaneous coronary interventions (PCI) often present unique challenges, frequently requiring larger guiding catheters (> 6F) to improve support, facilitate device exchange, accommodate larger burr size during rotational atherectomy, and allow for the simultaneous use of multiple devices.4-6 In some cases, patients may present with radial arteries that are too small to easily accommodate larger introducer sheaths, potentially increasing the risk of access site complications and radial artery occlusion (RAO).7,8 On the other hand, the use of large-bore catheters in TFA has been associated with an increased risk of access site bleeding and vascular complications.9-10
Recently, distal radial access (DRA) has emerged as an alternative to conventional TRA; it offers several advantages, including improved vascular outcomes with a significant reduction in RAO incidence,11 which may be attributed to a shorter hemostasis time and preservation of flow during hemostasis compression.12,13 However, the majority of DRA experience has been with introducer sheaths sized 6F or smaller, with limited data available for larger (> 6F) introducer sheaths/guiding catheters, which may lead to greater mismatch with the radial artery diameter and an increased risk of RAO.7,8 An intriguing aspect is that the size of the distal radial artery may be 0.5-mm smaller than at the wrist.14 Although a thin-walled sheath larger than 6F can be successfully inserted into the distal radial artery,15,16 it is not always possible to advance a guidewire larger than 6F without the use of special arterial tracking techniques.
Considering that even the slightest reduction in radial sheath diameter has been shown to significantly reduce the risk of RAO,17 Distal Rail-Tracking (DRT) had emerged as an innovative approach to complex PCI.18,19 In fact, DRT leverages DRA and optimizes the sheath/catheter-to-artery ratio by eliminating the need for an introducer sheath and instead utilizing the dedicated RAILWAY Sheathless Access System (Cordis) with a potential to further reduce the rate of RAO. This system allows for sheathless access with any available guiding catheter shape and improves its trackability in challenging forearm arterial anatomy,18,19 possibly reducing the need to crossover to TFA. The primary benefits of DRT are likely to preserve and facilitate the use of what is widely recognized as the safest vascular access approach: transradial. Therefore, this study aims to assess the feasibility, safety, and efficacy of DRT and to evaluate any practice differences resulting from its adoption as the primary strategy for the treatment of severely calcified disease by rotational atherectomy.
Methods
Study design and population
The SWITCH D-RIL (short-term outcomes and differences in clinical practice for severely calcified coronary artery disease treatment by rotational atherectomy after SWITCHing to Distal RaIL-tracking technique as the main vascular access) study is an investigator-driven, multicenter, ambispective cohort study designed to assess the performance and differences in clinical practice resulting from the adoption of DRT as the primary strategy for the treatment of severely calcified disease by rotational atherectomy.
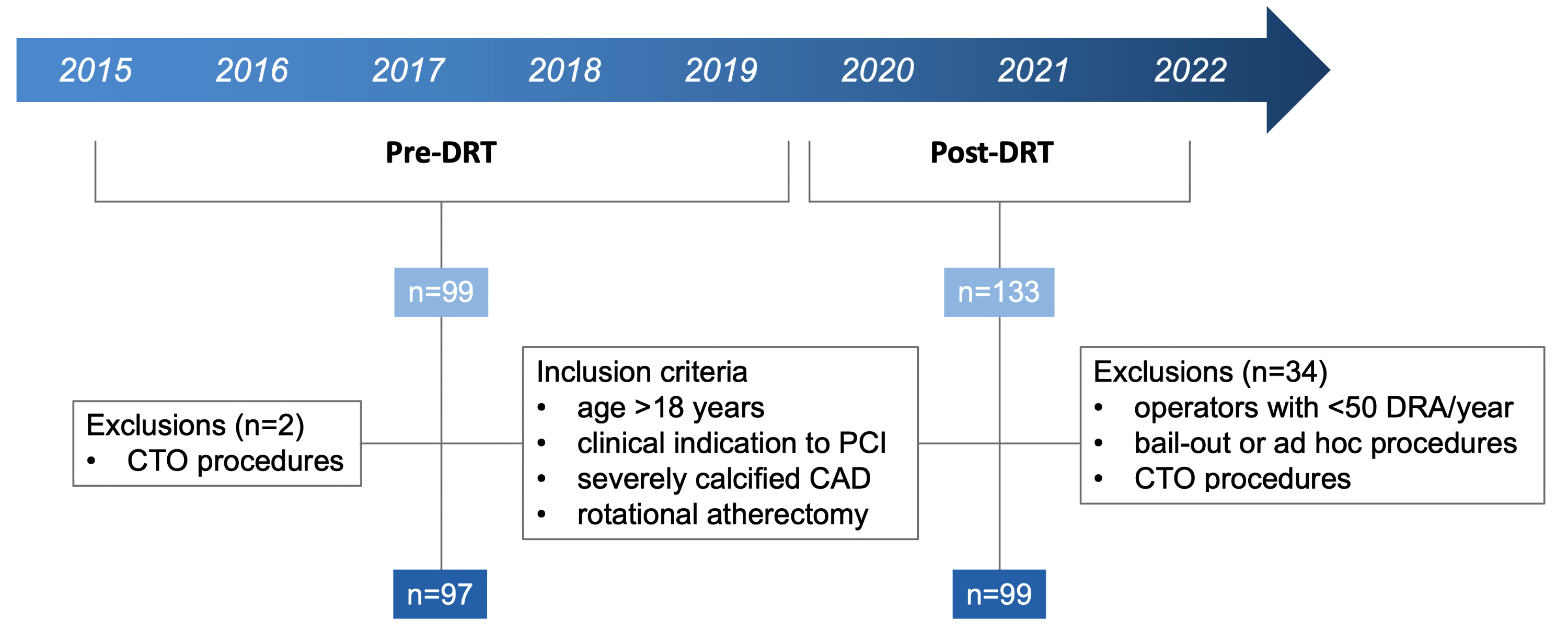
Consecutive patients 18 years or older with clinical indication to PCI who, according to clinical judgment, required treatment of severely calcified coronary disease by rotational atherectomy were eligible for enrollment. The study population was stratified into 2 groups based on the January 2020 implementation of DRT as the primary approach for complex PCI: pre-DRT and post-DRT. (Figure 1). For the pre-DRT group, which included cases from June 2015 to December 2019, only chronic total occlusion (CTO) procedures were excluded, resulting in a total of 97 patients. In the post-DRT group, 133 patients were enrolled from January 2020 to June 2022. In additon to the exclusion of CTO procedures, cases performed by operators with fewer than 50 complex DRA cases per year needing large-bore guiding catheters and bail-out or ad hoc procedures were excluded for this group; this was because the upfront DRT approach was intended only for elective cases. This resulted in a final cohort of 99 patients in the post-DRT group.
All patients included provided informed consent explicitly permitting the use of their anonymized data for research purposes in registries. The study complied with ethical standards and respected patient confidentiality in accordance with applicable regulations.
Study procedures
Starting in January 2020, 7F DRT was adopted as the first option for vascular access with the possibility to switch to conventional TRA or TFA using conventional introducer sheaths if deemed necessary by the operator according to the available clinical data, including known tortuosity of the arterial pathway, anatomical characteristics of the target lesion, and bleeding risk factors.
For DRA, ultrasound-guided puncture was used only if access could not be obtained within the first 60 seconds because it is known to improve procedural success of this more technically challenging access; for TFA, ultrasound-guided puncture was used systematically.
Rotational atherectomy was performed according to the best practice recommendations of the latest European consensus20 using the RotaPro rotational atherectomy system (Boston Scientific). No temporary transvenous pacing or central venous access was prepared, and transcoronary pacing21 was used in bail-out situations to manage any conduction abnormalities that might occur during the procedure. All procedures were performed by skilled and experienced manufacturer-certified operators with an overall volume of more than 300 PCIs per year.
Unfractionated heparin was administered according to patient weight (100 UI/kg), and anticoagulation levels were checked every 30 minutes to ensure an activated clotting time of greater than 300 seconds. Hemostasis was achieved with the Perclose ProGlide suture-mediated closure system (Abbott) followed by a light compression bandage in the case of TFA. An air-inflated device was used to achieve patent hemostasis after radial access: the TR Band (Terumo) for conventional TRA and the PreludeSYNC DISTAL (Merit Medical) for DRA.22 Lifelong low-dose aspirin was recommended for all patients in combination with either ticagrelor or clopidogrel for 6 to 12 months, depending on the clinical setting. In the case of concomitant chronic treatment with an oral anticoagulant, aspirin was usually discontinued after up to 3 months.
The RAILWAY Sheathless Access System
The RAILWAY Sheathless Access System enables direct access to the radial artery with a guiding catheter and eliminates the need for an introducer sheath, reducing the size of the arterial puncture by up to 2F compared with the use of a conventional introducer sheath. It consists of a long vessel dilator with a tapered tip and a lubricious hydrophilic coating in its distal 20 cm; it also provides a guidewire port at the appropriate distance from the tip for use with a short access guidewire. In addition to facilitating sheathless percutaneous delivery of a guidewire catheter from the skin through the subcutaneous tissue into the radial artery by eliminating the so-called “razor effect” through a smooth, atraumatic transition between the tip of the guidewire catheter and its tapered dilator, the RAILWAY Sheathless Access System also improves arterial trackability19 and hence crossing of complex anatomy.23
The RAILWAY Sheathless Access System is provided in a kit containing 2 dilators: one is compatible with a 0.021-inch guidewire aiming at an upfront sheathless access for elective PCI, and the other compatible with a 0.035-inch guidewire for conversion to a large-bore sheathless technique after initial use of a small introducer sheath and for quick exchange of guiding catheters. They are available in 5F (grey), 6F (green), and 7F (orange) versions to enable comatibilit with any existing guiding catheters. The kit also includes 2 needles (1 bare needle and 1 over-the-needle cannula) and a 45-cm long 0.021-inch guidewire.
The Distal RailTracking approach
To perform Distal RailTracking, a conventional 5F introducer sheath is first inserted into the distal radial artery and a 0.035-inch J-tip guidewire is positioned in the ascending aorta (Figure 2A). The 5F introducer sheath is then removed while applying finger pressure at the entry site to avoid unnecessary bleeding (Figure 2B), and a guiding catheter preloaded with the 0.035-inch-guidewire-compatible RAILWAY dilator (Figure 2C) is advanced over the 0.035-inch guidewire. Once the guidewire/RAILWAY dilator system reaches the subclavian artery, the RAILWAY dilator can be retrieved; this completes the sheathless approach (Figure 2D), and classic coronary engagement can be performed.
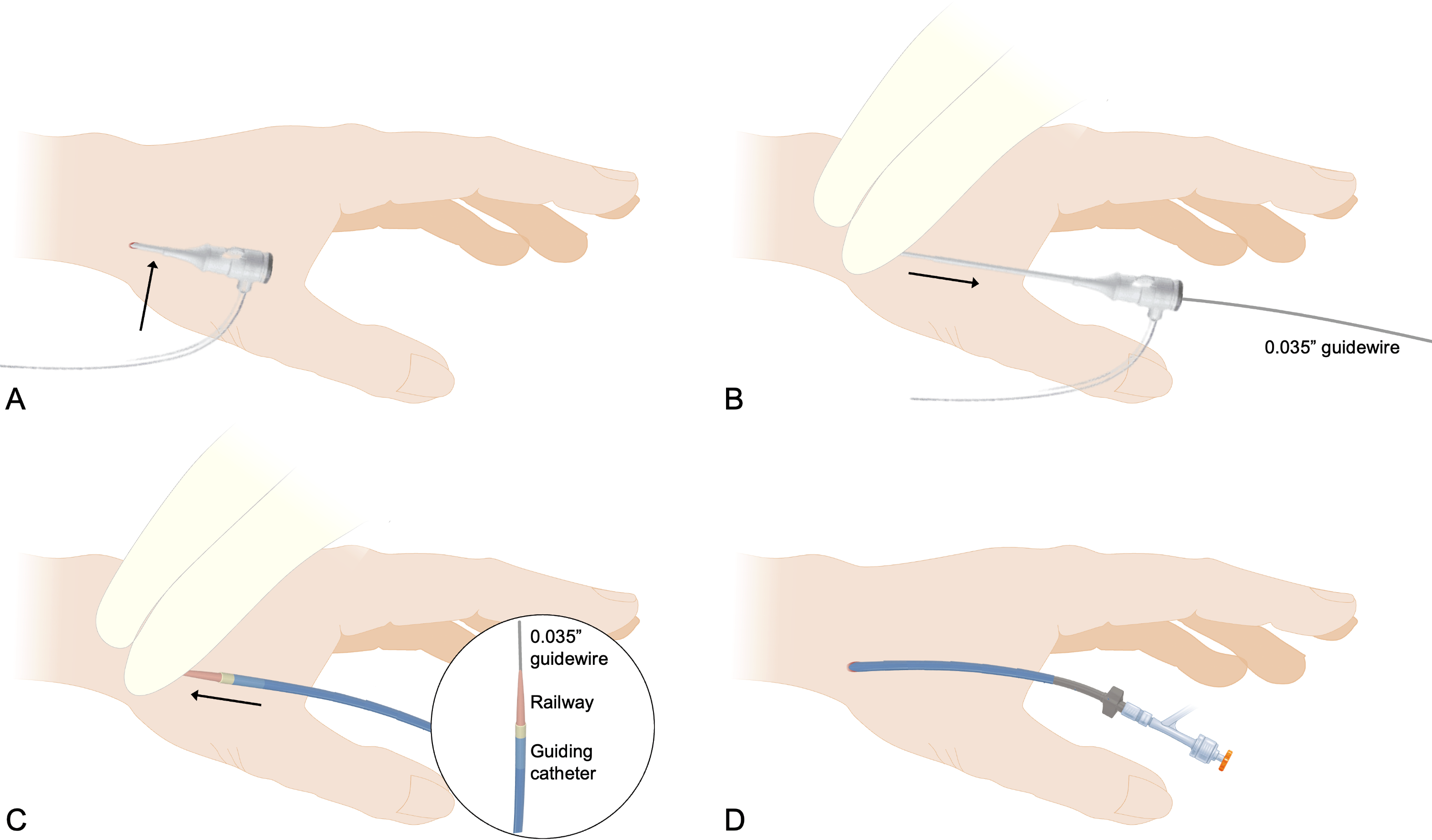
Compared with upfront sheathless access with the 0.021-inch-guidewire-compatible dilator, the preliminary use of a small introducer sheath has many advantages, including optimal administration of the spasmolytic cocktail before switching to the sheathless guiding catheter, increased support of the 0.035-inch guidewire, constant protection of the catheter end by the RAILWAY tapered tip, and the arterial tracking capabilities of the RAILWAY vessel dilator.
Study endpoints and definitions
The primary endpoint of the study was procedural success without access site crossover. Procedural success was defined as effective lesion preparation by rotational atherectomy followed by successful implantation of a drug-eluting stent with less than 10% residual stenosis and Thrombolysis in Myocardial Infarction (TIMI) III flow and no clinically relevant edge dissection. Secondary endpoints included the use of a guiding catheter sized 7F or larger as a "procedural facilitator" (facilitating the use of larger burr size, additional devices, and their rapid exchange), periprocedural complications (coronary perforation, wire fracture, burr fracture, hemodynamic instability), major adverse cardiovascular events (MACE) both in-hospital and at 30-day follow-up (MACE was defined as the event rate of all-cause mortality, cerebrovascular accident, or stent thrombosis), repeat target lesion revascularization, access site complication (clinically relevant bleeding events, non-occlusive injury, peripheral ischemia, cutaneous radial nerve injury), and non-access site clinically relevant bleeding according to the Bleeding Academic Research Consortium (BARC) criteria.24
Statistics
Continuous variables with a normal distribution according to the Shapiro-Wilk test were compared by Student’s t-test; otherwise the Mann-Whitney U test was used. Proportions were compared by Pearson’s chi-square test or Fisher’s exact test, as appropriate.
Data are reported as mean ± SD, or count (percentage) unless otherwise indicated. CIs for proportions were calculated using the Wilson method. A 2-sided P-value of less than 0.05 was required for statistical significance. Statistical analyses were performed with Stata release 17.0 (StataCorp).
Results
Patient population
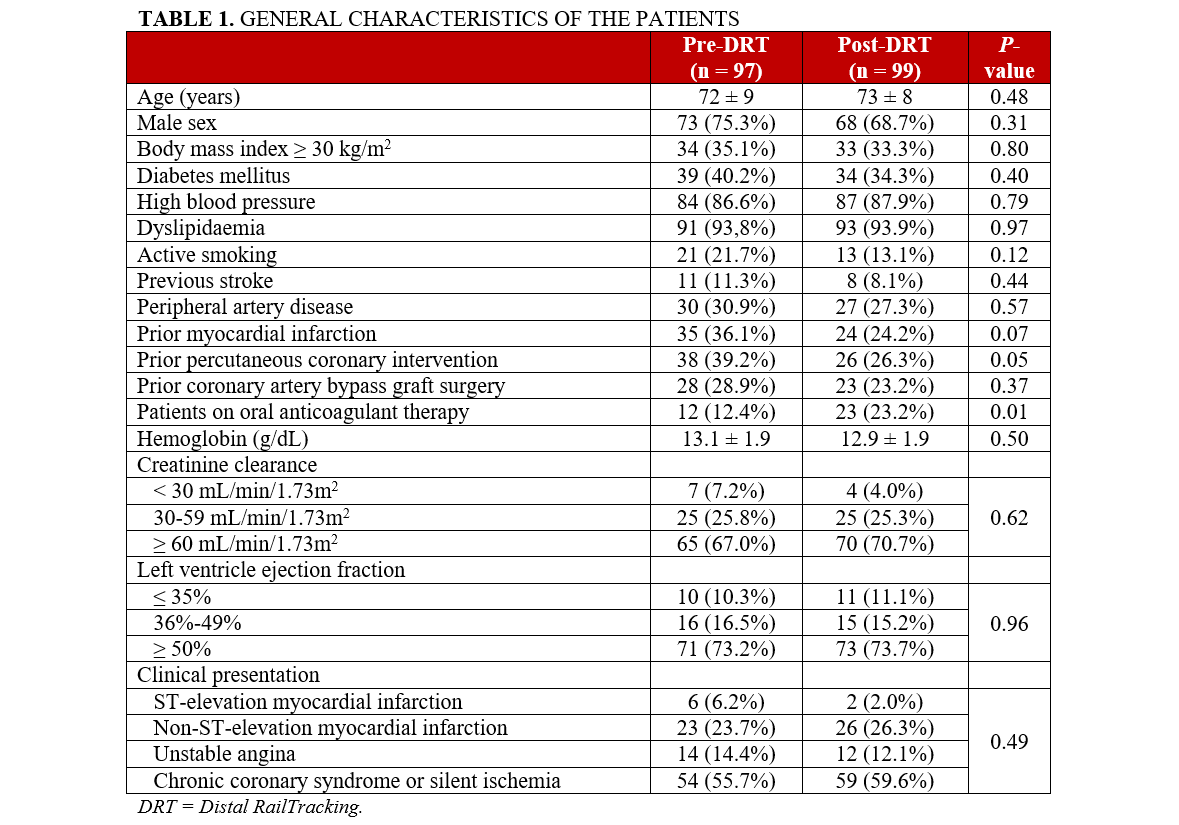
The general characteristics of the enrolled patients are summarized in Table 1 and reflect real -world conditions of this complex population. Acute coronary syndrome was the clinical presentation in 40.4% (40/99) of the patients in the post-DRT group and 44.3% (43/97) in the pre-DRT group. Except for a significant higher prevalence of oral anticoagulants in the post-DRT group (23.2% [23/99] vs 12.4% [12/97], P = .01), the general characteristics of the pre-DRT group were not significantly different from those of the post-DRT group, reflecting an overall homogeneous patient population.
Angiographic and procedural characteristics
As shown in Table 2, multivessel disease was present in more than half of the cases requiring a staged procedure: 69.5% (73/105) of the patients in the post-DRT group and 56.4%
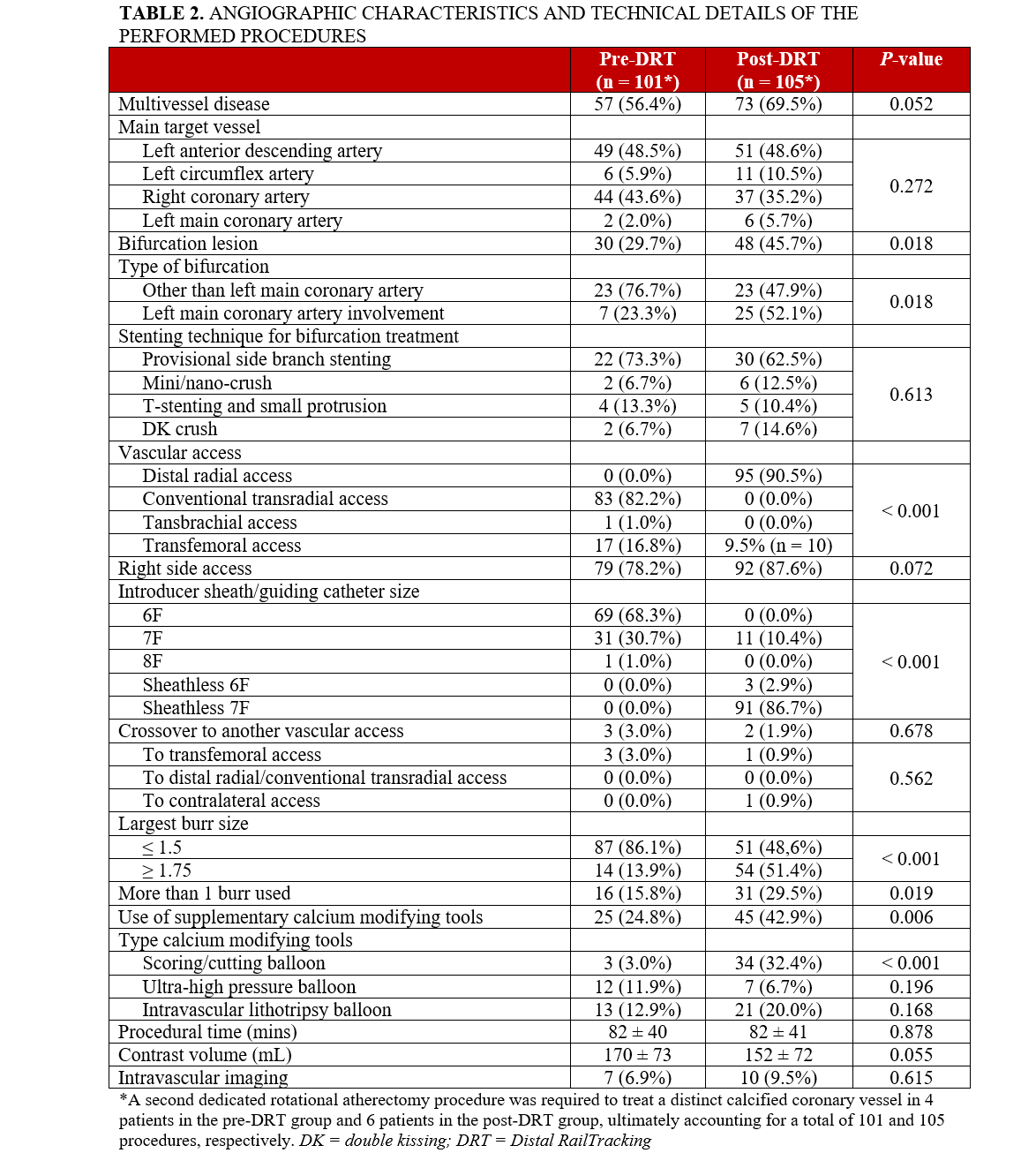
(57/101) of the patients in the pre-DRT group (P = .052). A second dedicated rotational atherectomy procedure was required to treat a distinct calcified coronary vessel in 6 patients in the post-DRT group and 4 patients in the pre-DRT group, for a total of 105 and 101 procedures, respectively. The most common target vessel was the left anterior descending artery. Approximately half of the calcified lesions in the post-DRT group involved a coronary bifurcation and were treated with rotational atherectomy of the calcified branch, most often followed by provisional side-branch stenting. Two-stent bifurcation techniques used for the remaining cases are detailed in Table 2.
In the post-DRT group, DRA was actually performed in 90.5% of cases (95/105), with the remaining patients undergoing TFA. In the pre-DRT group, conventional TRA was the most common access (82.2% [83/101]), followed by TFA (16.8% [17/101]). There was a single case of transbrachial access, an approach typically avoided because of its associated risks, particularly the serious potential for forearm ischemia from access site-related complications. There were very few crossovers to other vascular access in both groups. There was a trend toward more frequent right-side access in the post-DRT group (87.6% [92/105]) compared with the pre-DRT group (78.2% [79/101]).
In the post-DRT group, the use of a 7F sheathless guiding catheter was prevalent (86.7% [91/105]), but a 7F introducer sheath was used in 10.4% of cases (11/105), corresponding to all patients with TFA and 1 patient with DRA. In the pre-DRT group, a 6F introducer sheath was used in 68.3% of cases (69/101), while a 7F introducer sheath was used in 30.7% of cases (31/101). Overall, a guiding catheter sized 7F or larger was used in 97.1% (102/105) and 30.7% (31/101) of patients in the post- and pre-DRT groups, respectively (P < .001), with only one 8F guiding catheter being used exclusively in the pre-DRT group.
A burr sized 1.75 mm or larger was used more frequently in the post-DRT group than in the pre-DRT group (51.4% [54/105] vs 13.9% [14/101], P < .001), as was the use of calcium modifying devices (42.9% [45/105] vs 24.8% [25/101], P = .006), which was mainly driven by a higher use of scoring/cutting balloons (32.4% [34/105] vs 3.1% [3/101], P < .001).
Outcomes
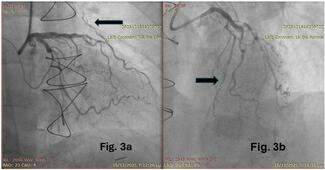
Procedural success and procedural success without need for access site crossover were high in both the post-DRT (98.1% [103/105] and 96.2% [101/105]) and pre-DRT (97.0% [98/101] and 94.1% [95/101]) groups, with no significant difference in in-hospital MACEs (0.9% [1/105] vs 1.0% [1/101], P = 1.0), coronary perforations (2.0% [2/105] vs 2.1% [2/101], P = 1.0), and BARC ≥ 3 bleeding (2.9% [3/105] vs 1.0% [1/101], P = .621), which were consistently low (Table 3, Figure 3). In the pre-DRT group, the reported MACE was related to cardiorespiratory arrest, with subsequent death in a very frail patient with severe left ventricular dysfunction and multivessel disease who required extracorporeal life support during the atherectomy procedure. The same patient also developed a severe femoral hematoma requiring transfusion. In the post-DRT group, the reported MACE was related to RotaWire (Boston Scientific) fracture, with the burr causing a severe left anterior descending artery perforation that could not be treated percutaneously and resulted in the patient’s death despite an attempt at bail-out surgical treatment.
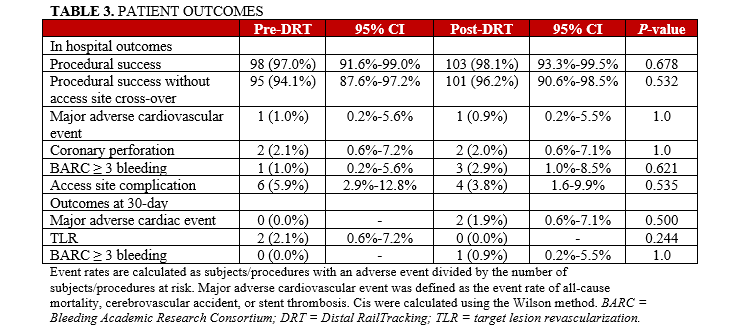
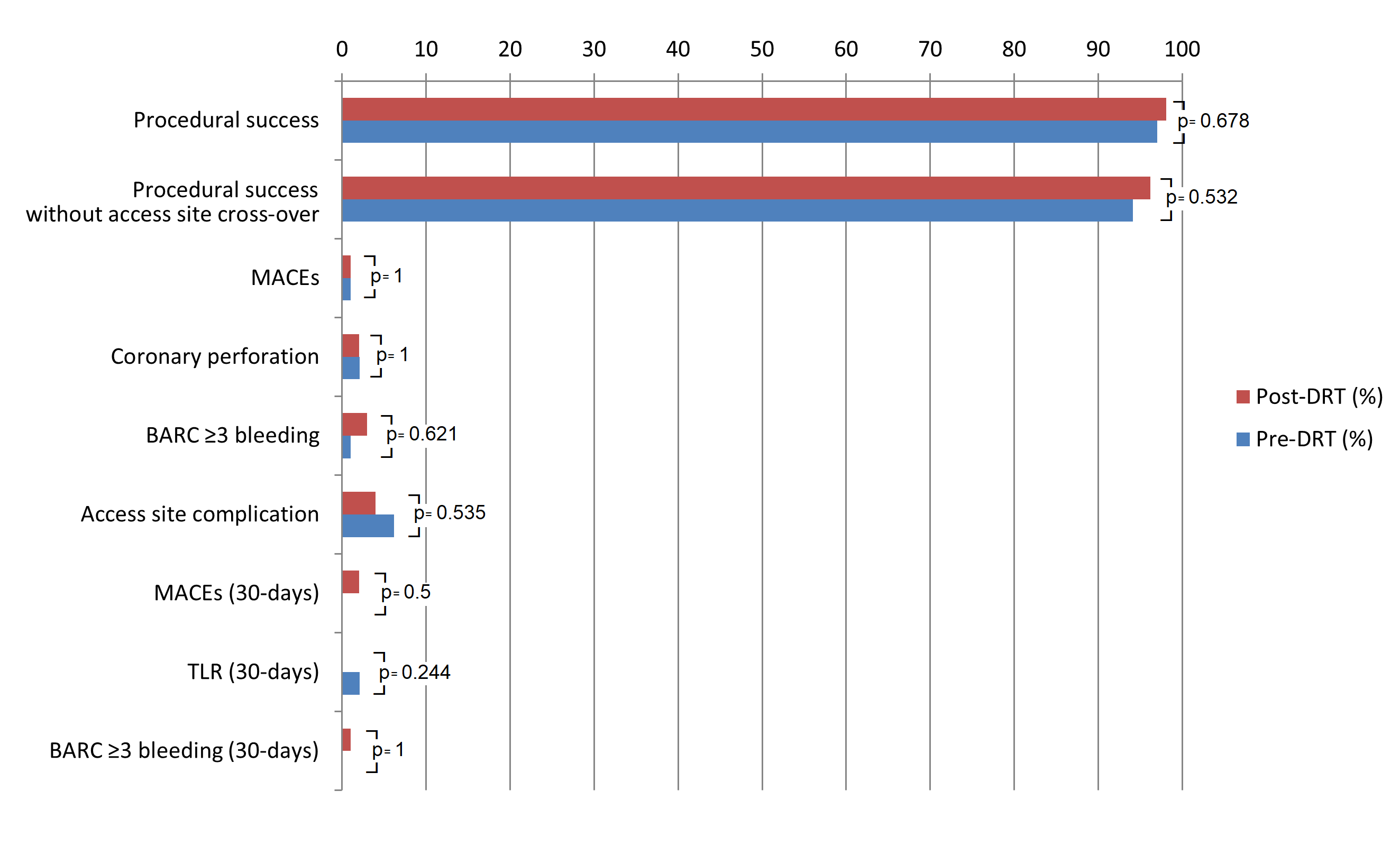
Major bleeding events occurred in 3 patients with severe rectal bleeding requiring transfusion and surgical ligation (n = 1), severe hematuria requiring transfusion and surgical resection of some bladder polyps (n = 1), and severe gastric bleeding treated by endoscopic electrocauterization (n = 1). Vascular access complications were not significantly different between the pre- and post-DRT groups (5.9% [6/101] vs 3.8% [4/105], P = .535). In the pre-DRT group, 5 patients developed a major hematoma at the femoral (n = 2) and radial (n = 3) arterial access sites, while another event was the aforementioned major bleeding after extravascular life support. In the post-DRT group, 2 patients developed significant hand hematoma and 2 patients developed pseudoaneurysms at the distal radial and femoral access sites, respectively. Both were effectively treated and resolved prior to patient discharge.
Similarly, 30-day outcomes were not significantly different between the pre- and post-DRT groups with respect to MACEs (0.0% [0/101] vs 1.9% [2/105], P = .480), repeat target lesion revascularization (2% [2/101] vs 0.0% [0/105], P = .480) and BARC ≥ 3 bleeding (0% [0/101] vs 0.9% [1/105], P = .970). The 2 patients with MACE in the post-DRT group each had a stent thrombosis related to discontinuation of antiplatelet therapy, one of which resulted in death in a patient who was noncompliant with his therapy. The other patient was on dual antiplatelet therapy but this was discontinued because of severe gastric bleeding.
Discussion
The SWITCH D-RIL study is a unique clinical study that comprehensively evaluated the clinical performance of DRT as a primary strategy for the treatment of severely calcified coronary artery disease by rotational atherectomy. It is also the largest multicenter, real-world registry to date focused on DRA for the treatment of complex coronary lesions.
Its key findings are (1) DRT is highly effective and associated with very low rates of MACE, major bleeding, and access site complications; (2) DRT is feasible in more than 90% of cases needing preparation with rotational atherectomy and has a very low rate of crossover to other vascular access; and (3) compared with a reference practice of prevalent conventional TRA and TFA in a lower proportion of cases, DRT achieves comparable efficacy and safety while allowing a significantly greater proportion of radial access and large-bore guiding catheters (Figure 4).
For years, many operators have preferred TFA for complex coronary lesions requiring large-bore catheters.4-6 This preference was reflected in the ROTATE registry, where TFA was predominantly chosen for PCI in cases with highly calcified lesions requiring rotational atherectomy (71.6%). In such cases, the 7F introducer sheath and guiding catheter size were the most commonly used (76.8%).25
Conversely, the recent Euro4C registry has shown how the interventional community has shifted to a predominantly TRA in daily clinical practice (71.8%), with the aim of reducing vascular complications but at the cost of reducing the size of the guiding catheter (75.1% of 6F guiding catheters) with all its potential multiple technical drawbacks.26 Nonetheless, 7F guiding catheters remained the choice in 24.9% of cases, often because of clinical considerations weighing the complexity of the disease and the patient's bleeding risk.
In this sense, the use of a 7F guiding catheter can be considered as a “procedural facilitator,” allowing for the use of larger burr size, additional devices, and, ultimately, their rapid exchange should any potential complication arise. This may lead to improved outcomes and enhanced procedural safety. However, this convenience may come at the cost of a higher incidence of vascular access site complications.7,9,10 Interestingly, an even more recent registry showed that TFA was preferred in 60% of cases, and the use of 7F introducer sheaths was significantly less common in the TRA group (59.1% TRA vs 76.6% TFA).27
If the goal is to minimize access site complications, DRA appears to be the most logical choice because it has demonstrated superior vascular outcomes compared with conventional TRA in randomized clinical trials.11 However, DRA has the potential limitation of a smaller size access site,14 so DRT seems to find its most rational use in this access. Undoubtedly, improving the technical feasibility and safety of using large-bore guiding catheters via DRA may further reduce the need for TFA and the subsequent risk of vascular complications. These considerations become even more valuable when addressing specific patient subpopulations, such as those at high bleeding risk, those with early coronary artery disease, or RAO in the opposite upper limb, where preserving radial access requires special attention. For these reasons, DRT represents a highly rational solution because it concurrently addresses the sheath/catheter-to-artery ratio, promotes the use of DRA, and facilitates the use of large-bore guiding catheters through this approach.
Our pre-DRT group shares similarities with the Euro4C registry, with a higher prevalence of TRA (82.2%) and 6F guiding catheters (68.3%), in line with current clinical practice trends. Conversely, the post-DRT group showed notable differences. In this group, 90.5% of cases involved DRA, with DRT being the predominant technique (89.6%). Notably, the cumulative prevalence of 7F guiding catheters, one of our secondary endpoints, increased to 97.2%, despite the extremely low rate of TFA.
This substantial shift may be attributed to the improved forearm crossing facilitated by DRT with large-bore catheters, thanks to its documented tracking capabilities.19 This advantage outperforms the use of a thin-walled introducer sheath15,16 or a child-and-mother strategy,28 both of which lack the seamless transition between the catheter tip and the 0.035-inch guidewire, a critical factor for the smooth advancement of large-bore guiding catheters. In addition, DRT offers greater support compared with the balloon-assisted tracking technique,29 which relies on a 0.014-inch guidewire and exhibits superior versatility compared with dedicated sheathless guiding catheter systems.30 Regardless of the sheathless technique used to obtain the vascular access, special attention must be given to the prevention of kinking of the guiding catheter at the skin entry point during manipulations. Strikingly, the higher prevalence of 7F guiding catheters in the post-DRT group significantly influenced our practice, allowing for the use of larger burrs (≥ 1.75 mm) in 51.4% of cases compared with 13.9% in the pre-DRT group (P < .001).
Despite a higher proportion of patients on oral anticoagulants in the post-DRT group and the associated increased bleeding risk, no significant differences were observed between the 2 groups in terms of access site complications and in-hospital and 30-day major bleeding events. This could be viewed as a hypothesis-generating result, suggesting that DRT might represent the safest approach in terms of access-site-related vascular complications, even in higher-risk populations. Moreover, the procedural success rates observed in our study are consistent with the high success rates documented in the literature and did not show significant differences between the pre- and post-DRT groups, nor did MACEs differ significantly between the groups.
It is also noteworthy that procedural success was achieved without a significant need for access site crossover. According to currently available evidence, one of the major limitations of DRA is its higher crossover rate when compared with conventional TRA. A recent meta-analysis reported an overall crossover rate of 12.5% for DRA compared with 3.8% for conventional TRA, with an odds ratio of 3.08.11 However, in our study population, the crossover rate was remarkably low, standing at 1.9%. This favorable outcome is likely due to our rigorous DRA puncture protocol, which strongly advocates the use of ultrasound guidance if an effective puncture is not achieved within the first 60 seconds.
Limitations
The main limitation of this study is its non-randomized design, which precludes the consideration of all potential confounding variables. Also, the ambispective design of our study did not allow for the assessment of variables not systematically recorded in patient records, such as RAO or coronary artery tortuosity. However, it is worth noting that our study population was highly selected, and the remarkable homogeneity observed between the 2 study periods does not affect the reliability of our findings. While our study convincingly reflects the adoption of a safer access strategy and a significantly lower rate of TFA use, despite a higher use of large-bore guiding catheters in the post-DRT group, it is important to acknowledge that our patient sample size may not be large enough to detect statistical differences in most secondary endpoints, which show a low prevalence in this specific patient population.25-27 This cohort study aims to complement the findings of randomized controlled trials and provide a diverse perspective that may either pave the way for innovative practices or, conversely, delineate an approach that requires reevaluation. Finally, the study was conducted by skilled operators with extensive experience with both rotational atherectomy and DRA. Therefore, the findings may not necessarily be reproducible by less experienced operators or in centers with lower procedural volumes.
Conclusions
Our study demonstrates that the DRT technique is feasible, safe, and effective for treating severely calcified coronary lesions. Utilizing a large-bore sheathless guiding catheter via DRA and the enhanced tracking of the RAILWAY device, DRT shows promise in improving outcomes across complex coronary interventions. Whenever large-bore guiding catheters are needed, such as for rotational atherectomy, sheathless DRA may be safely and effectively preferred over TFA. Further randomized clinical trials with larger populations are warranted to confirm these findings.
Affiliations and Disclosures
Giuseppe Colletti, MD1; MD; Gregory A. Sgueglia, MD, PhD2; Adrien Jossart, MD3; Caroline Lepièce, MD3; Olivier Gach, MD, PhD4; Alexandre Natalis, MD1; Laura Peter, MD1; Silviu Dumitrascu, MD3; Claudiu Ungureanu, MD3
From the 1Cardiology Department, Groupe Vivalia, Clinique Saint-Joseph, Arlon, Belgium; 2Cardiology Department, Sant’Eugenio Hospital, Rome, Italy; 3Cardiology Department, Jolimont Hospital, La Louvière, Belgium; 4Cardiology Department, CHC MontLégia, Liège, Belgium.
Disclosures: Dr Colletti has received consulting fees from PHILIPS and honoraria for educational events from Abbott and Boston Scientific, outside of the submitted work. Dr Sgueglia has received consulting and lecture fees from Terumo, Cordis and GE Healthcare, outside of the submitted work. Dr Natalis has received consulting fees from Boston Scientific and support for attending meetings from Boston Scientific and Abbott, outside of the submitted work. Dr Ungureanu has received consulting fees from Boston Scientific and Cordis, outside of the submitted work. The remaining authors report no financial relationships or conflicts of interest regarding the content herein.
Address for correspondence: Giuseppe Colletti, MD, Cardiology Department, Groupe Vivalia, Clinique Saint-Joseph, Rue des Déportés 137, 6700 Arlon, Belgium. Email: giucol85@gmail.com
References
- Neumann FJ, Sousa-Uva M, Ahlsson A, et al; ESC Scientific Document Group. 2018 ESC/EACTS guidelines on myocardial revascularization. Eur Heart J. 2019;40(2):87-165. doi:10.1093/eurheartj/ehy394
- Lawton JS, Tamis-Holland JE, Bangalore, et al. 2021 ACC/AHA/SCAI guideline for coronary artery revascularization: a report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines. J Am Coll Cardiol. 2022;79(2):e21-e129. doi:10.1016/j.jacc.2021.09.006
- Gargiulo G, Giacoppo D, Jolly SS, et al; Radial Trialists’ Collaboration. Effects on mortality and major bleeding of radial versus femoral artery access for coronary angiography or percutaneous coronary intervention: meta-analysis of individual patient data from 7 multicenter randomized clinical trials. Circulation. 2022;146(18):1329-1343. doi:10.1161/CIRCULATIONAHA.122.061527
- Chung S, Her SH, Song PS, Song YB, et al. Trans-radial versus trans-femoral intervention for the treatment of coronary bifurcations: results from Coronary Bifurcation Stenting registry. J Korean Med Sci. 2013;28(3):388-395. doi:10.3346/jkms.2013.28.3.388
- Maeremans J, Walsh S, Knaapen P, et al. The hybrid algorithm for treating chronic total occlusions in europe: the RECHARGE registry. J Am Coll Cardiol. 2016;68(18):1958-1970. doi:10.1016/j.jacc.2016.08.034
- Watt J, Austin D, Mackay D, Nolan J, Oldroyd KG. Radial versus femoral access for rotational atherectomy: a UK observational study of 8622 patients. Circ Cardiovasc Interv. 2017;10(12):e005311. doi:10.1161/CIRCINTERVENTIONS.117.005311
- Uhlemann M, Möbius-Winkler S, Mende M, et al. The Leipzig prospective vascular ultrasound registry in radial artery catheterization: impact of sheath size on vascular complications. JACC Cardiovasc Interv. 2012;5(1):36-43. doi:10.1016/j.jcin.2011.08.011
- Saito S, Ikei H, Hosokawa G, Tanaka S. Influence of the ratio between radial artery inner diameter and sheath outer diameter on radial artery flow after transradial coronary intervention. Catheter Cardiovasc Interv. 1999;46(2):173-178. doi:10.1002/(SICI)1522-726X(199902)46:2<173::AID-CCD12>3.0.CO;2-4
- Grossman PM, Gurm HS, McNamara R, et al; Blue Cross Blue Shield of Michigan Cardiovascular Consortium (BMC2). Percutaneous coronary intervention complications and guide catheter size: bigger is not better. JACC Cardiovasc Interv. 2009;2(7):636-644. doi:10.1016/j.jcin.2009.05.012
- Meijers TA, Aminian A, van Wely M, et al. Randomized comparison between radial and femoral large-bore access for complex percutaneous coronary intervention. JACC Cardiovasc Interv. 2021;14(12):1293-1303. doi:10.1016/j.jcin.2021.03.041
- Ferrante G, Condello F, Rao SV, et al. Distal vs conventional radial access for coronary angiography and/or intervention: a meta-analysis of randomized trials. JACC Cardiovasc Interv. 2022;15(22):2297-2311. doi:10.1016/j.jcin.2022.09.006
- Sgueglia GA, Santoliquido A, Gaspardone A, Di Giorgio A. First results of the distal radial access doppler study. JACC Cardiovasc Imaging. 2021;14(6):1281-1283. doi:10.1016/j.jcmg.2020.11.023
- Pacchioni A, Mugnolo A, Sanz Sanchez J, et al. Radial artery occlusion after conventional and distal radial access: impact of preserved flow and time-to-hemostasis in a propensity-score matching analysis of 1163 patients. Catheter Cardiovasc Interv. 2022;99(3):827-835. doi:10.1002/ccd.30005
- Norimatsu K, Kusumoto T, Yoshimoto K, et al. Importance of measurement of the diameter of the distal radial artery in a distal radial approach from the anatomical snuffbox before coronary catheterization. Heart Vessels. 2019;34(10):1615-1620. doi:10.1007/s00380-019-01404-2
- Aminian A, Iglesias JF, Van Mieghem C, et al. First prospective multicenter experience with the 7 French Glidesheath slender for complex transradial coronary interventions. Catheter Cardiovasc Interv. 2017;89(6):1014-1020. doi:10.1002/ccd.26773
- Gasparini GL, Garbo R, Gagnor A, Oreglia J, Mazzarotto P. First prospective multicentre experience with left distal transradial approach for coronary chronic total occlusion interventions using a 7 Fr Glidesheath Slender. EuroIntervention. 2019;15(1):126-128. doi:10.4244/EIJ-D-18-00648
- Aminian A, Saito S, Takahashi A, et al. Comparison of a new slender 6 Fr sheath with a standard 5 Fr sheath for transradial coronary angiography and intervention: RAP and BEAT (Radial Artery Patency and Bleeding, Efficacy, Adverse evenT), a randomised multicentre trial. EuroIntervention. 2017;13(5):e549-e556. doi:10.4244/EIJ-D-16-00816
- Colletti G, Auslender J, De Meester A, Aminian A, Kayaert P, Ungureanu C. Feasibility and safety of performing complex coronary interventions by distal radial artery using the Railway Sheathless Vascular System. J Invasive Cardiol. 2020;32(12):459-462. doi:10.25270/jic/20.00201
- Ungureanu C, Gasparini GL, Aminian A, et al. RailTracking: A novel technique to overcome difficult anatomy during transradial approach. J Invasive Cardiol. 2022;34(11):E757-E762. doi:10.25270/jic/22.00049
- Barbato E, Carrié D, Dardas P, et al; European Association of Percutaneous Cardiovascular Interventions. European expert consensus on rotational atherectomy. EuroIntervention. 2015;11(1):30-36. doi:10.4244/EIJV11I1A6
- Kusumoto H, Ishibuchi K, Hasegawa K, Otsuji S. Trans-coronary pacing via Rota wire prevents bradycardia during rotational atherectomy: a case report. Eur Heart J Case Rep. 2022;6(2):ytac013. doi:10.1093/ehjcr/ytac013
- Pancholy S, Coppola J, Patel T, Roke-Thomas M. Prevention of radial artery occlusion-patent hemostasis evaluation trial (PROPHET study): a randomized comparison of traditional versus patency documented hemostasis after transradial catheterization. Catheter Cardiovasc Interv. 2008;72(3):335-340. doi:10.1002/ccd.21639
- Ungureanu C, Carlier S, Ghafari C, Auslender J, De Meester de Ravestein A, Colletti G. Successful crossing of complex radial and brachial artery anatomy using a new approach: Railtracking. Am J Case Rep. 2022;23:e934760. doi:10.12659/AJCR.934760
- Mehran R, Rao SV, Bhatt DL, et al. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation. 2011;123(23):2736-2747. doi:10.1161/CIRCULATIONAHA.110.009449
- Kawamoto H, Latib A, Ruparelia N, et al. In-hospital and midterm clinical outcomes of rotational atherectomy followed by stent implantation: the ROTATE multicentre registry. EuroIntervention. 2016;12(12):1448-1456. doi:10.4244/EIJ-D-16-00386
- Bouisset F, Barbato E, Reczuch K, et al. Clinical outcomes of PCI with rotational atherectomy: the European multicentre Euro4C registry. EuroIntervention. 2020;16(4):e305-e312. doi:10.4244/EIJ-D-19-01129
- Ferstl P, Drentwett AS, Bargon S, et al. Rotational atherectomy via the transradial access: success rates, procedural parameters and complications. Heart Vessels. 2022;37(9):1478-1488. doi:10.1007/s00380-022-02053-8
- Patel T, Shah S, Pancholy S. "Combo" technique for the use of 7F guide catheter system during transradial approach. Catheter Cardiovasc Interv. 2015;86(6):1033-1040. doi:10.1002/ccd.26119
- Patel T, Shah S, Pancholy S, Rao S, Bertrand OF, Kwan T. Balloon-assisted tracking: a must-know technique to overcome difficult anatomy during transradial approach. Catheter Cardiovasc Interv. 2014;83(2):211-220. doi:10.1002/ccd.24959
- Mamas M, D'Souza S, Hendry C, et al. Use of the sheathless guide catheter during routine transradial percutaneous coronary intervention: a feasibility study. Catheter Cardiovasc Interv. 2010;75(4):596-602. doi:10.1002/ccd.22246