Orthotopic Transcatheter Tricuspid Valve Replacement in a Patient With Carcinoid Syndrome
© 2025 HMP Global. All Rights Reserved.
Any views and opinions expressed are those of the author(s) and/or participants and do not necessarily reflect the views, policy, or position of the Journal of Invasive Cardiology or HMP Global, their employees, and affiliates.
Carcinoid heart disease (CHD) occurs in up to 60% of patients with carcinoid syndrome and primarily involves the right-sided heart valves. Severe tricuspid regurgitation (TR) is the hallmark of CHD, often resulting in debilitating right heart failure. Surgical valve replacement has traditionally been the definitive therapy, but many patients are inoperable because of hepatic dysfunction or advanced disease. Transcatheter tricuspid valve replacement (TTVR) is an emerging option for high-risk patients. Here, we present unique images visualizing the native anatomy of a patient’s tricuspid valve (TV) annulus, a massive TR, and, eventually, an orthotopic TTVR with a dedicated device.
A 90-year-old woman with a history of metastatic small intestinal neuroendocrine tumor presented with worsening dyspnea, abdominal distention, and lower extremity edema, for which she was treated with somatostatin analogs and telotristat. Cardiac involvement was suspected after the development of progressive signs of right heart failure. An echocardiogram revealed massive TR (vena contracta: 30 x 34 mm; effective regurgitant orifice area: 2.7 cm2; regurgitant volume: 96 mL; and hepatic flow reversal), dilated right-sided chambers (right ventricular [RV] end-diastolic volume: 152 mL; RV end-systolic volume: 86 mL), and preserved right- and left-sided function (RV ejection fraction: 43.6%; fractional area change: 39%; left ventricular ejection fraction: 65%) (Figure A-C, Videos 1-4).
The patient was evaluated by the multidisciplinary heart team, hepatology, and oncology. Given her age, frailty, and metastatic disease, she was deemed high risk for surgery (EuroSCORE II: 4.37%; TriScore: 22%). Computed tomography measurements showed the TV to be multi-scalloped with general tethering, and the septal leaflet appeared short, plastered in the midportion, and flailing anteriorly. Based on these findings, the LuX-Valve Plus transcatheter TV platform (Jenscare Scientific Co. Ltd.) was deemed suitable for the patient.
TTVR with a LuX-Valve Plus has been described elsewhere.1 Briefly, under general anesthesia with fluoroscopic and transesophageal echocardiogram (TEE) guidance, a 30/55 LuX-Valve Plus was successfully deployed across the native tricuspid annulus via the right jugular vein (33F delivery system). After adjusting the delivery system to be in the center and perpendicular to the tricuspid annulus, the “rabbit-ears” graspers (Figure D) were released and the anchor tongue was fired onto the ventricular septum (Figure E), stabilizing the platform. The delivery system was then removed.
Post-deployment imaging confirmed a good valve position, elimination of the TR, and a preserved RV function (Figure F) (Videos 5 and 6). Repeat TTE before discharge showed a well-seated bioprosthesis with no residual TR and improved RV dimensions. The patient was alive and doing well 3 months post-TTVR.
Orthotopic TTVR is a viable and effective option in patients with CHD and massive TR who are poor surgical candidates. Multidisciplinary evaluation is essential, and dedicated TV systems may offer durable results in select patients.
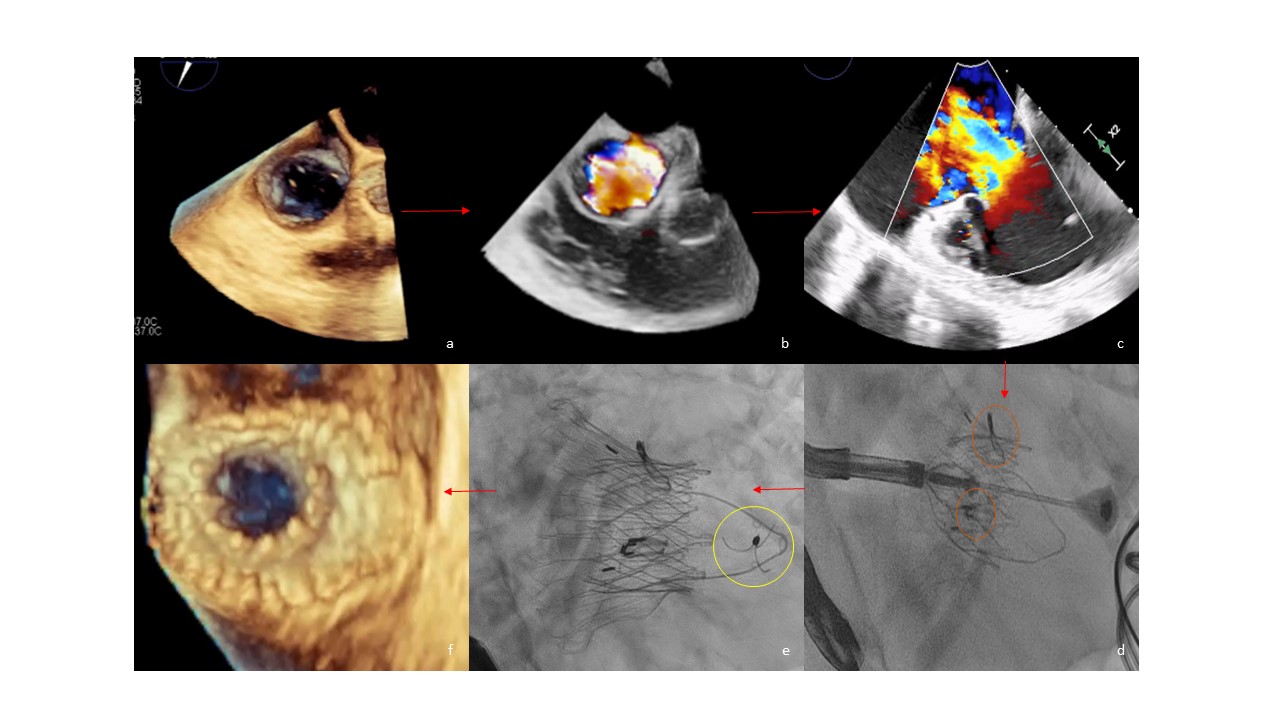
Figure. (A) Transesophageal echocardiogram (TEE) 3-dimensional (3D) image at 65o of the tricuspid valve annulus (area 15.2 cm2). (B) TEE image with 3D color Doppler showing the massive tricuspid regurgitation (TR). (C) TEE image at 0o depicting the massive TR. (D) The 2 ‘rabbit-ears’ graspers are seen clipping the leaflets (orange circles). (E) The LuX-Valve Plus (Jenscare Scientific Co. Ltd.) is released and the anchor has attached in the septum (yellow circle). (F) TEE 3D image at 70o showing the well-opposed prosthetic valve.
Affiliations and Disclosures
Konstantinos Stathogiannis, MD, FACC; Michalis Chrissoheris, MD; Konstantinos Spargias, MD
From the Transcatheter Heart Valves Department, Hygeia Hospital, Athens, Greece.
Disclosures: The authors report no financial relationships or conflicts of interest regarding the content herein.
Consent statement: The authors confirm that informed consent was obtained from the patient for this study described in the manuscript and to the publication, including any and all images.
Address for correspondence: Konstantinos Stathogiannis, MD, FACC, 9 Erythrou Stavrou Street, Marousi 151 23, Greece. Email: kstathog@hotmail.com
References
- Zhang Y, Lu F, Li W, et al. A first-in-human study of transjugular transcatheter tricuspid valve replacement with the LuX-Valve Plus system. EuroIntervention. 2023;18(13):e1088-e1089. doi:10.4244/EIJ-D-22-00517




















