Adaptive Use of a Large-Bore Sheath to Access the Right Ventricular Outflow Tract in a Pediatric Patient
© 2026 HMP Global. All Rights Reserved.
Any views and opinions expressed are those of the author(s) and/or participants and do not necessarily reflect the views, policy, or position of the Journal of Invasive Cardiology or HMP Global, their employees, and affiliates.
J INVASIVE CARDIOL 2026. doi:10.25270/jic/25.00393. Epub January 6, 2026.
Given the limited availability of pediatric-specific equipment, interventional cardiologists frequently adapt devices originally designed for adult use when treating congenital heart disease.1,2 Access challenges have been further compounded by recent updates to the European Medical Device Regulation, which have reduced access to devices for orphan and pediatric indications.3,4
We report the case of a 9-year-old boy (weight: 30 kg) with repaired tetralogy of Fallot who underwent complete surgical correction with a GORE-TEX Cardiovascular Patch (W. L. Gore & Associates, Inc.) at 5 months old. On follow-up, transthoracic echocardiography demonstrated mild pulmonary stenosis with severe regurgitation, and his right ventricular ejection fraction had declined to 42%. In light of progressive valve dysfunction, staged percutaneous pulmonary valve implantation was planned.
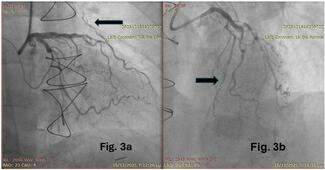
During the first stage, a Lunderquist Extra-Stiff Wire Guide (Cook Medical) was positioned in the distal right pulmonary artery (RPA). Advancement of a 64-cm 16F Sentrant sheath (Medtronic) was hindered by the patient’s size and anatomy. To overcome this, a controlled loop was intentionally created within the right atrium, allowing the sheath to advance smoothly into the RPA without kinking or deformation (Video). An AndraStent XXL 30 (Andramed) was then deployed on a 24 × 40-mm Andra2Balloon (Andramed), successfully establishing a stable landing zone for valve implantation (Figure).
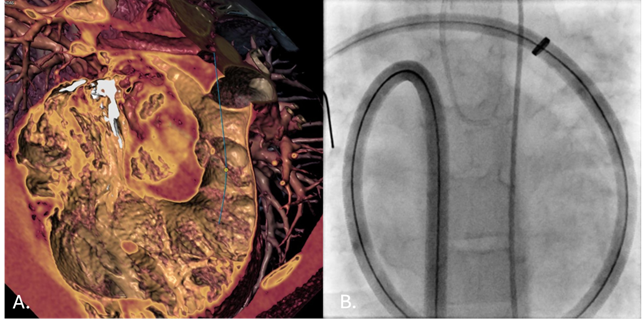
The patient tolerated the procedure well. A few weeks later, a 26-mm SAPIEN 3 valve (Edwards Lifesciences) was subsequently implanted during the second stage, thereby completing the intervention.
This case highlights how strategic adaptation of adult interventional devices—specifically, looping a large-bore sheath within the right atrium—can overcome anatomic challenges in pediatric patients and facilitate complex transcatheter procedures, despite ongoing constraints posed by regulatory frameworks and limited device availability.
Affiliations and Disclosures
Guste Cesnaite, MD1,2; Ugne Stulpinaite, MD1,2; Sigitas Cesna, MD, PhD1,2
From the 1Centre of Cardiology and Angiology, Vilnius University Hospital Santaros Klinikos, Vilnius, Lithuania; 2Clinic of Cardiac and Vascular Diseases, Institute of Clinical Medicine, Faculty of Medicine, Vilnius University, Vilnius, Lithuania.
Disclosures: The authors report no financial relationships or conflicts of interest regarding the content herein.
Consent statement: The authors confirm that informed consent was obtained from the patient for the intervention described in the manuscript and to the publication.
Address for correspondence: Ugne Stulpinaite, MD, Vilnius University Hospital Santaros Klinikos, Vilnius, Lithuania. Email: ugne.stulpinaite@gmail.com
References
1. Peiris V, Xu K, Agler HL, et al. Children and adults with rare diseases need innovative medical devices. J Med Device. 2018;12(3):0347011-347018. doi:10.1115/1.4040489
2. Melvin T, Dooms MM, Koletzko B, et al. Orphan and paediatric medical devices in Europe: recommendations to support their availability for on-label and off-label clinical indications. Expert Rev Med Devices. 2024;21(10):893-901. doi:10.1080/17434440.2024.2404257
3. Driesen BW, Warmerdam EG, Sieswerda GJ, et al. Percutaneous pulmonary valve implantation: current status and future perspectives. Curr Cardiol Rev. 2019;15(4):262-273. doi:10.2174/1573403X1566618122411385
4. Melvin T, Kenny D, Gewillig M, Fraser AG. Orphan medical devices and pediatric cardiology - what interventionists in Europe need to know, and what needs to be done. Pediatr Cardiol. 2023;44(2):271-279. doi:10.1007/s00246-022-03029-1