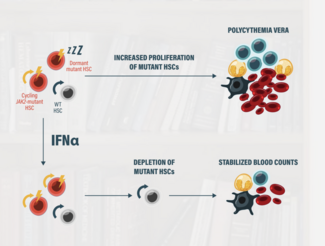
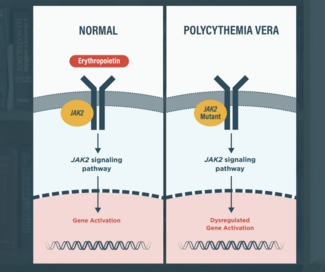
BESREMi (ropeginterferon alfa-2b) in the Treatment of Polycythemia Vera (PV): A Closer Look at the Long-Term Data
Learn about the long-term efficacy and safety of BESREMi (ropeginterferon alfa-2b), and review practical strategies for managing side effects in clinical practice.

Ellen K. Ritchie, MD, is an assistant professor of medicine and a member of the Leukemia Program at the Weill Cornell Medical College of Cornell University and the New York Presbyterian Hospital in New York City. Dr Ritchie graduated from Barnard College at Columbia University and received her medical degree from the College of Physicians and Surgeons at Columbia University in New York City, where she was also elected to the Alpha Omega Alpha Honor Society. She completed her internship and residency in internal medicine and her fellowship in hematology and medical oncology at New York Presbyterian Hospital, Columbia campus. Dr Ritchie's research interests are in the treatment of older patients with anemia, cytopenias, myelodysplastic syndromes, myeloproliferative disorders, and acute leukemia. She is interested in finding better therapies and supportive care strategies for older patients, particularly those with hematologic malignancies. Dr Ritchie is the principal investigator on clinical trials investigating new diagnostic techniques, supportive care strategies, and therapeutics aimed at the older patient. She collaborates with investigators in the Division of Geriatrics and Gerontology. Additionally, she has been the author or co-author of many publications.
Transcript
My name is Ellen Ritchie, and I'm associate professor of clinical medicine at Weill Cornell Medical College in New York City. I am the assistant director of the leukemia program. I treat mainly myeloproliferative neoplasms, polycythemia vera, essential thrombocythemia, and primary myelofibrosis.
Welcome to the final video of our series as we explore the advancements in the treatment landscape for polycythemia vera, or PV.
Today, we will focus on a key treatment option, BESREMi (ropeginterferon alfa-2b), and its long-term efficacy and safety, particularly as compared to another cytoreductive therapy option, hydroxyurea (HU). We will also review practical strategies for managing side effects in clinical practice.
Indications1
BESREMi is indicated for the treatment of adults with polycythemia vera.
Boxed Warning1
WARNING: RISK OF SERIOUS DISORDERS
Interferon alfa products may cause or aggravate fatal or life-threatening neuropsychiatric, autoimmune, ischemic, and infectious disorders. Patients should be monitored closely with periodic clinical and laboratory evaluations. Therapy should be withdrawn in patients with persistently severe or worsening signs or symptoms of these conditions. In many, but not all cases, these disorders resolve after stopping therapy.
Dosage and Administration1
Recommended starting dose is 100
Increase the dose by 50
Let’s review the 2 trials that supported the use of BESREMi as a long-term treatment option for patients with PV.
The PROUD-PV study was a phase 3, 12-month, open-label, randomized study evaluating the efficacy and safety of BESREMi compared to hydroxyurea in adult patients with PV.2
It included 257 patients with confirmed PV and JAK2 mutation, 127 of whom were randomly assigned to receive either BESREMi or hydroxyurea for 12 months.2 BESREMi was administered subcutaneously biweekly at a starting dose of 100
The primary efficacy endpoint was a composite outcome combining complete hematologic response (CHR), defined as hematocrit <45%, no phlebotomy in the preceding 3 months, platelets ≤400 x 109/L, and leukocytes ≤10 x 109/L, and normal spleen size by imaging at 12 months.2
One of the secondary endpoints of the PROUD-PV study was a change in JAK2 V617F allelic burden to assess the treatment’s molecular response.
Twenty-one percent of patients who received BESREMi and 28% of patients who received hydroxyurea achieved a complete hematologic response with normal spleen size at 12 months. The primary endpoint of non-inferiority was not achieved.2
Building on the PROUD-PV study, CONTINUATION-PV assessed the long-term efficacy and safety of BESREMi for 6 years. At the end of 12 months of PROUD-PV, 171 of the patients who completed PROUD-PV rolled into the CONTINUATION-PV study. Ninety-five of the 127 patients from the PROUD-PV BESREMi group continued BESREMi treatment, while 76 of the 127 patients who received hydroxyurea in the PROUD-PV received the best available treatment during the CONTINUATION-PV study.2
The coprimary efficacy endpoints of CONTINUATION-PV were the proportion of patients who achieved a complete hematologic response and normal spleen size and a complete hematologic response with improved disease burden.2
The limitations of CONTINUATION-PV included that it was initially designed as a single-arm extension of PROUD-PV. The second arm with hydroxyurea or best available treatment was subsequently added for comparison, resulting in a study entry gap of approximately 21 weeks.3
In terms of efficacy, the proportion of patients who achieved a complete hematologic response was significantly higher with BESREMi than with hydroxyurea.
Notably, the proportion of patients with a treatment response to BESREMi increased gradually up to 12 to 24 months and was sustained at 36 months.2
At 72 months, the proportion of BESREMi-treated patients achieving complete hematologic response was 72.6%, and 81.4% of patients treated with BESREMi required no phlebotomies to maintain hematocrit below 45%.3
In a post-hoc analysis, BESREMi demonstrated a significant improvement in event-free survival when compared to standard treatment, with risk events being defined as thromboembolic events, disease progression, or death. Median event-free survival was not reached by the end of the study, and this analysis was not prespecified.4
Combined, these long-term data underscore BESREMi’s value in clinical practice.
Now, let’s review the safety and tolerability data from the PROUD and CONTINUATION-PV studies.
Eight percent of BESREMi-treated patients discontinued due to drug-related toxicity. In the standard therapy group across both studies, 4% of patients discontinued because of drug-related toxicity.2
The most common adverse events reported in >10% of patients treated with BESREMi included hematologic side effects, including thrombocytopenia, leukopenia, and anemia, liver function abnormalities, including increased γ-glutamyltransferase, alanine aminotransferase, and aspartate aminotransferase, and fatigue, headache, arthralgia, and dizziness.2
The proportion of patients with serious adverse events, adverse events of grade 3 or higher, and major thromboembolic adverse events were all similar between the 2 treatment groups.2
Treatment-related serious adverse events occurred in 2% of the patients in the BESREMi group and 4% of the patients in the hydroxyurea group.2
The most frequently reported grade 3 and grade 4 treatment-related adverse events were increased γ-glutamyltransferase and increased alanine aminotransferase in the BESREMi group, and leukopenia and thrombocytopenia in the standard therapy group.2
Together, the safety data from the PROUD and CONTINUATION-PV studies show that BESREMi was manageable in patients with PV, and the frequency of treatment-related adverse events was comparable with that of hydroxyurea.
To manage adverse reactions, consider dose modification or interruption. The recommended dose modification protocols for liver enzyme elevation, with or without concomitant bilirubin elevation, or other evidence of hepatic decompensation, cytopenia, and depression, are presented here.1
Providers are advised to monitor complete blood counts (CBC) every 2 weeks during the titration phase and dose modification phase, and every 3 to 6 months during the maintenance phase. More frequent monitoring of CBC may be necessary for clinically indicated patients.1
Today, we reviewed the long-term clinical trial data showing that BESREMi induced higher hematologic and clinical responses when compared to the standard therapy with hydroxyurea. BESREMi offers durable responses and a manageable safety profile, making it an important therapeutic option to consider for patients with PV.
Thank you for joining us!
References:
- BESREMi. Prescribing information. PharmaEssentia USA Corporation; 2024.
- Gisslinger H, Klade C, Georgiev P, et al. Ropeginterferon alfa-2b versus standard therapy for polycythaemia vera (PROUD-PV and CONTINUATION-PV): a randomised, non-inferiority, phase 3 trial and its extension study. Lancet Haematol. 2020;7(3):e196-e208. doi:10.1016/S2352-3026(19)30236-4
- PharmaEssentia. Additional clinical results. BESREMI website. Updated January 2025. Accessed March 20, 2025. https://www.besremihcp.com/additional-clinical-efficacy/
- Gisslinger H, Klade C, Georgiev P, et al. Event-free survival in patients with polycythemia vera treated with ropeginterferon alfa-2b versus best available treatment. Leukemia. 2023;37(10):2129-2132. doi:10.1038/s41375-023-02008-6
- Data on file. PharmaEssentia Corporation.