BESREMi (ropeginterferon alfa-2b) as a Treatment Option for Low- and High-Risk Adult Patients With Polycythemia Vera (PV)
Review the limitations of historic treatment options for polycythemia vera and learn about ropeginterferon alfa-2b-njft (BESREMi), a novel treatment option.

Ellen K. Ritchie, MD, is an assistant professor of medicine and a member of the Leukemia Program at the Weill Cornell Medical College of Cornell University and the New York Presbyterian Hospital in New York City. Dr Ritchie graduated from Barnard College at Columbia University and received her medical degree from the College of Physicians and Surgeons at Columbia University in New York City, where she was also elected to the Alpha Omega Alpha Honor Society. She completed her internship and residency in internal medicine and her fellowship in hematology and medical oncology at New York Presbyterian Hospital, Columbia campus. Dr Ritchie's research interests are in the treatment of older patients with anemia, cytopenias, myelodysplastic syndromes, myeloproliferative disorders, and acute leukemia. She is interested in finding better therapies and supportive care strategies for older patients, particularly those with hematologic malignancies. Dr Ritchie is the principal investigator on clinical trials investigating new diagnostic techniques, supportive care strategies, and therapeutics aimed at the older patient. She collaborates with investigators in the Division of Geriatrics and Gerontology. Additionally, she has been the author or co-author of many publications.
Transcript
My name is Ellen Ritchie, and I'm associate professor of clinical medicine at Weill Cornell Medical College in New York City. I am the assistant director of the leukemia program. I treat mainly myeloproliferative neoplasms, polycythemia vera, essential thrombocythemia, and primary myelofibrosis.
Welcome to this video series on polycythemia vera, or PV, and its evolving treatment landscape.
Today, we will explore the limitations of historic treatment options for PV, introduce ropeginterferon alfa-2b-njft (BESREMi) as a novel treatment option, and discuss its recent designation as a preferred first-line cytoreductive therapy in the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Myeloproliferative Neoplasms.1
PV is a chronic myeloproliferative neoplasm characterized by an overproduction of red blood cells, often accompanied by an increase in white blood cells and platelets. The elevated blood cell counts increase blood viscosity, raising the risk of thrombosis, cardiovascular complications, and long-term complications, such as myelofibrosis (MF) and acute myeloid leukemia (AML).2 Polycythemia vera is associated with a high symptom burden that can significantly impair quality of life, even in early or low-risk stages of the disease. Symptoms include fatigue, pruritus, and night sweats, which arise from cytokine dysregulation, early satiety and abdominal discomfort due to splenomegaly, and microvascular symptoms, such as headache, dizziness, and erythromelalgia.3-5
Mutations in the Janus tyrosine kinase 2 (JAK2), particularly in JAK2 V617F, are found in nearly all cases of PV and lead to procoagulant changes, elevating the risk for thrombosis.6,7
The primary goals of PV management are to decrease hematocrit levels to below 45%, reduce thrombosis risk, and delay disease progression.6
Historically, the management of PV has relied on 3 main approaches – phlebotomy, low-dose aspirin, and cytoreductive therapy.3
Low-dose aspirin alone or with phlebotomy is recommended for low-risk PV patients, patients who are younger than 60 years of age and no history of thrombosis. 3
While it is a mainstay in PV management, in my clinical experience, I have seen that phlebotomy can lead to iron deficiency, which has been associated with worsening of disease-related symptoms.8
Additionally, it requires frequent office visits and may be unpleasant for many patients. Moreover, phlebotomy does not offer stable hematocrit control, as the hematocrit can rise between phlebotomies and result in the exacerbation of symptoms.3,9,10
For high-risk patients, those over 60 years of age and/or a history of thrombosis, and symptomatic low-risk patients, cytoreductive therapy is recommended.11
The traditional first-line choice for cytoreductive therapy has been hydroxyurea, a chemotherapy agent that suppresses the production of blood cells in the bone marrow.12
Although it is effective at reducing hematocrit in the short-term, what I have seen over years of treating patients with PV is that long-term use of hydroxyurea is associated with potential toxicities and complications, including neutropenia and macrocytic anemia, oral and leg ulcers, and skin lesions, and it may lead to resistance or intolerance. Additionally, hydroxyurea has been suggested to increase the risk of leukemic transformation, although this remains highly controversial.3,6,13,14
Given these limitations, there is a need for a treatment option that can provide lasting hematologic control.6
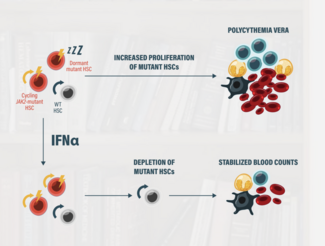
Interferon alfa (IFN𝛼) has been reported to have a disease-modifying ability by selectively targeting malignant stem cells and inducing long-term molecular remission in some patients with myeloproliferative neoplasms, making it a potentially preferred treatment option to use early in the course of the disease. However, the toxicity associated with early versions of IFN𝛼 has limited its use.2
BESREMi is a long-acting, mono-pegylated interferon that utilizes a novel approach to treating PV.2
Indications15
BESREMi is indicated for the treatment of adults with polycythemia vera.
Boxed Warning15
WARNING: RISK OF SERIOUS DISORDERS
Interferon alfa products may cause or aggravate fatal or life-threatening neuropsychiatric, autoimmune, ischemic, and infectious disorders. Patients should be monitored closely with periodic clinical and laboratory evaluations. Therapy should be withdrawn in patients with persistently severe or worsening signs or symptoms of these conditions. In many, but not all cases, these disorders resolve after stopping therapy.
Dosage and Administration15
Recommended starting dose is 100 𝜇g by subcutaneous injection every 2 weeks (50 𝜇g if receiving hydroxyurea).
Increase the dose by 50 𝜇g every 2 weeks (up to a maximum of 500 𝜇g) until the hematologic parameters) are stabilized. Interrupt or discontinue dosing according to the full Prescribing Information if certain adverse reactions occur.
BESREMi has an extended half-life, which allows 1 subcutaneous dosing every 2 weeks.2,15
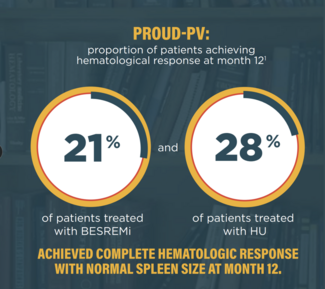
BESREMi induced complete hematologic response in 80% and comprehensive disease control in 61% of patients treated in a pivotal trial that led to its US Food and Drug Administration approval.15
It demonstrated long-term disease control, with the duration of response extending beyond 7.5 years in a clinical trial.16
Moreover, in a post hoc analysis, BESREMi demonstrated a significant improvement in the probability of event-free survival compared to standard cytoreductive therapy. This analysis was not prespecified.17
BESREMi demonstrated a favorable safety and tolerability profile, with zero cases of AML and 1 case of myelofibrosis over the 7.5-year clinical trial.18
These therapeutic benefits of ropeginterferon alfa-2b-njft (BESREMi) led to its inclusion as a preferred first-line cytoreductive option in the NCCN Guidelines® for the treatment of PV. The National Comprehensive Cancer Network® (NCCN®) recently updated its recommendations to recognize ropeginterferon alfa-2b-njft as a preferred first-line cytoreductive therapy for both high-risk and symptomatic low-risk patients.1
For high-risk patients, BESREMi offers potential long-term therapeutic benefits. For symptomatic low-risk patients experiencing new thrombosis or disease-related major bleeding, splenomegaly, progressive thrombocytosis and/or leukocytosis, or disease-related symptoms such as pruritus, night sweats, and fatigue, as well as those requiring frequent phlebotomy or unable to tolerate it, ropeginterferon alfa-2b-njft (BESREMi) is a recommended cytoreductive therapy option.1,5
In conclusion, BESREMi is a novel cytoreductive therapy for patients with PV that provides sustained hematologic response. Combined with its favorable tolerability, BESREMi is a treatment option that should be considered for high-risk and low-risk patients with PV with disease-associated symptoms.
Thank you for joining me today! I encourage you to review the NCCN Guidelines and consider how this treatment option might fit into your clinical practice. To learn more, please watch the other videos in the series.
References:
- Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Myeloproliferative Neoplasms V.1.2025. © National Comprehensive Cancer Network, Inc. 2025. All rights reserved. Accessed March 2, 2025. To view the most recent and complete version of the guideline, go online to NCCN.org. NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way.
- Gisslinger H, Klade C, Georgiev P, et al. Ropeginterferon alfa-2b versus standard therapy for polycythaemia vera (PROUD-PV and CONTINUATION-PV): a randomised, non-inferiority, phase 3 trial and its extension study. Lancet Haematol. 2020;7(3):e196-e208. doi:10.1016/S2352-3026(19)30236-4
- Griesshammer M, Gisslinger H, Mesa R. Current and future treatment options for polycythemia vera. Ann Hematol. 2015;94(6):901-910. doi:10.1007/s00277-015-2357-4
- Emanuel RM, Dueck AC, Geyer HL, et al. Myeloproliferative neoplasm (MPN) symptom assessment form total symptom score: prospective international assessment of an abbreviated symptom burden scoring system among patients with MPNs. J Clin Oncol. 2012;30(33):4098-4103. doi:10.1200/JCO.2012.42.3863
- Cuthbert D, Stein BL. Polycythemia vera-associated complications: pathogenesis, clinical manifestations, and effects on outcomes. J Blood Med. 2019;10:359-371. doi:10.2147/JBM.S189922
- Iurlo A, Cattaneo D, Bucelli C, Baldini L. New perspectives on polycythemia vera: from diagnosis to therapy. Int J Mol Sci. 2020;21(16):5805. doi:10.3390/ijms21165805
- Harvard Medical School. Cancer drug takes fast track to clinical trials. April 18, 2008. Accessed March 20, 2025. https://hms.harvard.edu/news/cancer-drug-takes-fast-track-clinical-trials
- Ginzburg YZ, Feola M, Zimran E, et al. Dysregulated iron metabolism in polycythemia vera: etiology and consequences. Leukemia. 2018;32(10):2105-2116. doi:10.1038/s41375-018-0207-9
- Grossi G. Polycythemia vera management: addressing the burden of symptoms and phlebotomy dependence. AMJC. November 5, 2024. Accessed April 12, 2025. https://www.ajmc.com/view/polycythemia-vera-management-addressing-the-burden-of-symptoms-and-phlebotomy-dependence
- PharmaEssentia. Clinician’s guide. BESREMI website. Updated 2025. Accessed April 21, 2025. https://www.besremihcp.com/wp-content/uploads/2025/02/BESREMi-Core-Visual-Aid-CVA-Digital-2.pdf
- Barbui T, Vannucchi AM, De Stefano V, et al. Ropeginterferon versus standard therapy for low-risk patients with polycythemia vera. NEJM Evid. 2023;2(6):EVIDoa2200335. doi:10.1056/EVIDoa2200335
- Kapor S, Vukotić M, Subotički T, et al. Hydroxyurea induces bone marrow mesenchymal stromal cells senescence and modifies cell functionality in vitro. J Pers Med. 2021;11(11):1048. doi:10.3390/jpm11111048
- Finazzi G, Barbui T. How I treat patients with polycythemia vera. Blood. 2007;109(12):5104-5111. doi:10.1182/blood-2006-12-038968
- Bewersdorf JP, How J, Masarova L, et al. Moving toward disease modification in polycythemia vera. Blood. 2023;142(22):1859-1870. doi:10.1182/blood.2023021503
- BESREMi. Prescribing information. PharmaEssentia USA Corporation; 2024.
- PharmaEssentia. PEGINVERA results. BESREMI website. Updated January 2025. Accessed March 20, 2025. https://www.besremihcp.com/peginvera-clinical-efficacy/
- Gisslinger H, Klade C, Georgiev P, et al. Event-free survival in patients with polycythemia vera treated with ropeginterferon alfa-2b versus best available treatment. Leukemia. 2023;37(10):2129-2132. doi:10.1038/s41375-023-02008-6
- PharmaEssentia. Safety. BESREMI website. Updated 2025. Accessed March 20, 2025.