Prognostic Implications Over Time of Platelet FcɣRIIa Expression in Patients With Myocardial Infarction: A Secondary Analysis
© 2025 HMP Global. All Rights Reserved.
Any views and opinions expressed are those of the author(s) and/or participants and do not necessarily reflect the views, policy, or position of the Journal of Invasive Cardiology or HMP Global, their employees, and affiliates.
Abstract
Objectives. In patients with myocardial infarction (MI), quantifying platelet FcɣRIIa (pFCG) stratifies the risk of subsequent MI, stroke, and death. The authors conducted a secondary analysis to assess the prognostic implications of the pFCG test over the course of 1 year after MI.
Methods. Patients (n = 764) hospitalized for type 1 MI (ST elevation and non-ST elevation) were enrolled in a prospective non-interventional trial. Inclusion criteria included at least 2 of the following: age 65 years or older, multivessel coronary artery disease, prior MI, chronic kidney disease, and diabetes mellitus. Flow cytometry was used to quantify pFCG at a core laboratory. High and low pFCG were defined by a prespecified threshold. The primary endpoint (n = 98) was the composite of MI, stroke, and death.
Results. The time-to-first-event analysis demonstrated that the pFCG test had the greatest prognostic power early after MI. The hazard ratio (HR) for the primary composite endpoint in all subjects was greatest during the first month (3.84, P = .0009), and the HR for the first 6 months was 2.90 (P = .00005). Similar trends were apparent for patients treated with percutaneous coronary intervention and those treated with medical therapy alone. Analysis of components of the primary endpoint, the composite of MI and death, as well as MI alone, showed similar trends.
Conclusions. The pFCG test is a powerful prognostic marker of ischemic risk during the first 6 months after MI. The prognostic information provided by the pFCG test should be useful to clinicians as they balance risk of ischemic events with that of bleeding to define a treatment strategy.
Introduction
The use of dual antiplatelet therapy (DAPT) that combines aspirin plus an oral P2Y12 inhibitor reduces the risk of early and late thrombotic events such as myocardial infarction (MI).1-3 DAPT increases the risk of bleeding, an effect that is more pronounced with prolonged exposure. For example, the STOPDAPT-2 ACS (Short and Optimal Duration of Dual Antiplatelet Therapy After Everolimus-Eluting Cobalt-Chromium Stent-2 in acute coronary syndrome) trial demonstrated reduced bleeding (hazard ratio [HR] 0.46; 95% CI, 0.23-0.94) in patients treated with shortened DAPT.4 Both bleeding and ischemic events (such as MI) are prognostically important.5 Thus, a risk-informed, individualized approach to treatment has the potential to reduce both bleeding and ischemic events.6,7
In 2 prior prospective non-interventional studies, we found that the quantification of platelet FcɣRIIa (pFCG) discriminates patients at higher and lower risk of subsequent ischemic cardiovascular events.8,9 The pFCG test was initially evaluated in a single-center study in patients with MI,8 and the results were validated in a 25-center study with 800 patients.9 In the multicenter study, the primary composite endpoint (MI, stroke, death) occurred more frequently in patients with high pFCG (HR 2.09; 95% CI, 1.34-3.26; P = .001). Notably, the pFCG test was particularly effective in identifying patients at higher and lower risk of recurrent MI (HR 3.24; 95% CI, 1.64-6.37; P = .001).
FcɣRIIa amplifies the activation of platelets.10,11 Thus, greater platelet expression of FcɣRIIa increases platelet reactivity12 and promotes thrombosis.13 Megakaryocyte production of FcɣRIIa determines platelet expression and is driven by interferon ɣ.14 Accordingly, pFCG will be influenced by the inflammatory state of an individual patient. Because the inflammatory state can change over time after MI,15 the objective of this secondary analysis of the previously described multicenter study9 is to assess the prognostic implications of the pFCG test over the course of 1 year after MI.
Methods
Participants
The study design has been previously reported.9 Adults (age > 18) were enrolled in a prospective observational (non-interventional study). Sites were allowed to use their institutional review board or a central review board (WCG). Patients were enrolled after providing informed consent during hospitalization for type 1 MI (ST elevation or non-ST elevation). To ensure a sufficient number of primary endpoints were accrued, the inclusion criteria required that participants had at least 2 of the following: age 65 years or older, multivessel coronary artery disease, prior MI, chronic kidney disease (defined as estimated glomerular filtration rate [eGFR] ˂ 60 mL/min/1.73 m2), or diabetes mellitus. Patients were excluded if they were enrolled in another trial in which they may receive anticoagulant or antiplatelet treatment as part of the trial intervention, and when non-cardiovascular conditions, in the judgment of the investigator, would limit survival to less than 2 years.
Quantification of pFCG
Citrate anticoagulated blood was taken at each clinical site within 2 weeks of enrollment. At each site, platelets were fixed with formaldehyde within 24 hours after phlebotomy. Subsequently, samples were shipped to the core laboratory and processed within 5 days of fixation. Analytic studies demonstrated that pFCG expression was stable for that interval. The pFCG test quantifies FcɣRIIa on the surface of platelets with the use of flow cytometry. Platelets were identified by size and the presence of a platelet marker (CD42b conjugated with phycoerythin-CY5 [BD Biosciences]). FcɣRIIa was detected with 5G1, which was labeled in a 1:1 molar ratio with phycoerythrin. Flow cytometry output (mean fluorescence intensity) was converted to molecules of pFCG/platelet with the use of standardized beads (Quantibrite; BD Biosciences). A prespecified threshold (1750 molecules/platelet) was used to identify high and low pFCG.
Outcomes
The primary endpoint was a composite of MI, stroke, and all-cause death. A secondary endpoint was the incidence of clinically significant bleeding according to the Bleeding Academic Research Consortium (BARC) scale (types 2-5).16 Telephone follow-up was performed every 6 months and used a standardized questionnaire. Patient-reported events were confirmed by medical record review. Investigators identified clinical events (MI, stroke) by objective findings that included biomarkers (MI) or imaging (stroke).
Statistics
The trial design was event driven. Enrolled patients (~800) were planned to be followed until at least 80 ischemic events had occurred and the last subject had completed 18 months of follow-up. This analysis focused on events during the first year of follow-up.
A Cox proportional hazards model was used to compare patients with high and low pFCG with respect to the primary endpoint and secondary endpoints that included components of the primary endpoint. The dependent variable was the number of days from enrollment to the occurrence of a primary endpoint, with censoring at the 1-year follow-up. Independent variables included pFCG, age in years, history of diabetes mellitus, prior revascularization, multivessel coronary artery disease defined as 2 or more vessels or left main with a stenosis of at least 50%, chronic kidney disease defined as an eGFR of less than 60 mL/min/1.73 m2, prior MI, hypertension, tobacco use, previous stroke or transient ischemic attack, and peripheral arterial disease. The proportional hazards assumption was tested with the use of Schoenfeld residuals and found to be non-significant, indicating that the assumption was satisfied. The Cox proportional hazards model was used to assess risk of the pFCG as a continuous measure. In this case, the HR provided the multiplicative change in risk per unit change in pFCG. This analysis was performed in 2 ways: (1) by dividing pFCG values by 1000 (HR is the multiplicative increase in risk per each 1000-unit increase in pFCG), and (2) using the base 2 logarithm of pFCG (HR is the multiplicative increase in risk per doubling of pFCG). Significance was defined as a P-value of less than 0.05.
Results
Management strategies in the multicenter study included percutaneous coronary intervention (PCI) (63%), medical management alone (22%), and coronary artery bypass graft (CABG) (15%). The number of ischemic events among patients treated with CABG (n = 120 patients with 10 primary endpoints) was not sufficient to make conclusions; therefore, subgroup analysis was confined to patients treated with PCI and medical management alone. Similarly, we observed a small number of strokes (4 events) as primary events, so analysis of components of the primary endpoint was confined to MI and death.
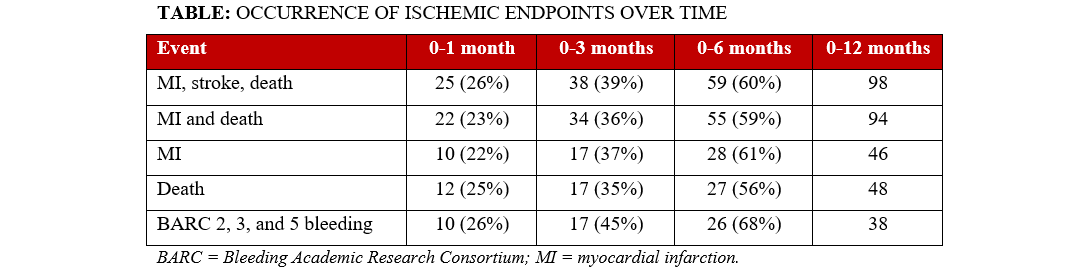
During the first year after MI, 98 patients experienced a primary endpoint (MI, stroke, or death) and 38 experienced BARC 2, 3, or 5 bleeding. The occurrence of endpoints was more common earlier: approximately 25% occurred during the first month, approximately 39% during the first 3 months, and approximately 60% during the first 6 months (~ 40% during the second 6 months). A similar trend was apparent for death and MI, MI alone, death alone, and bleeding (Table).
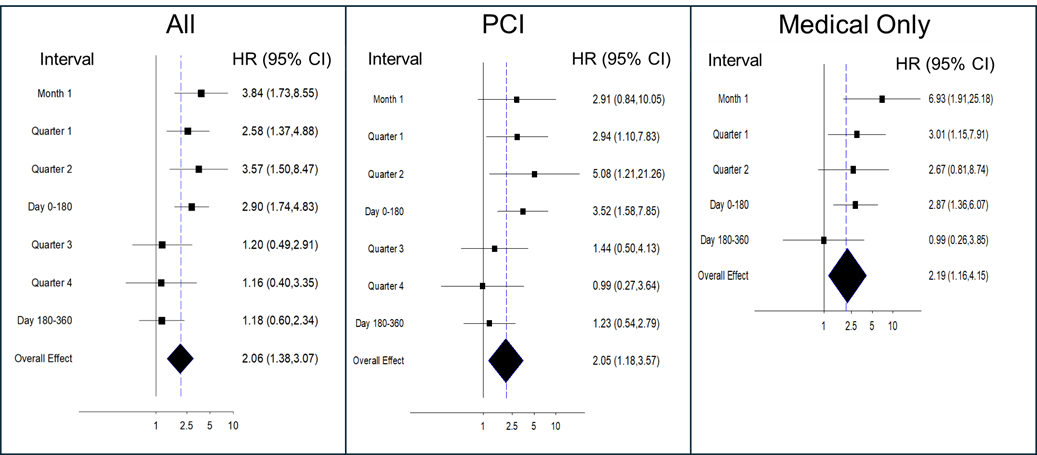
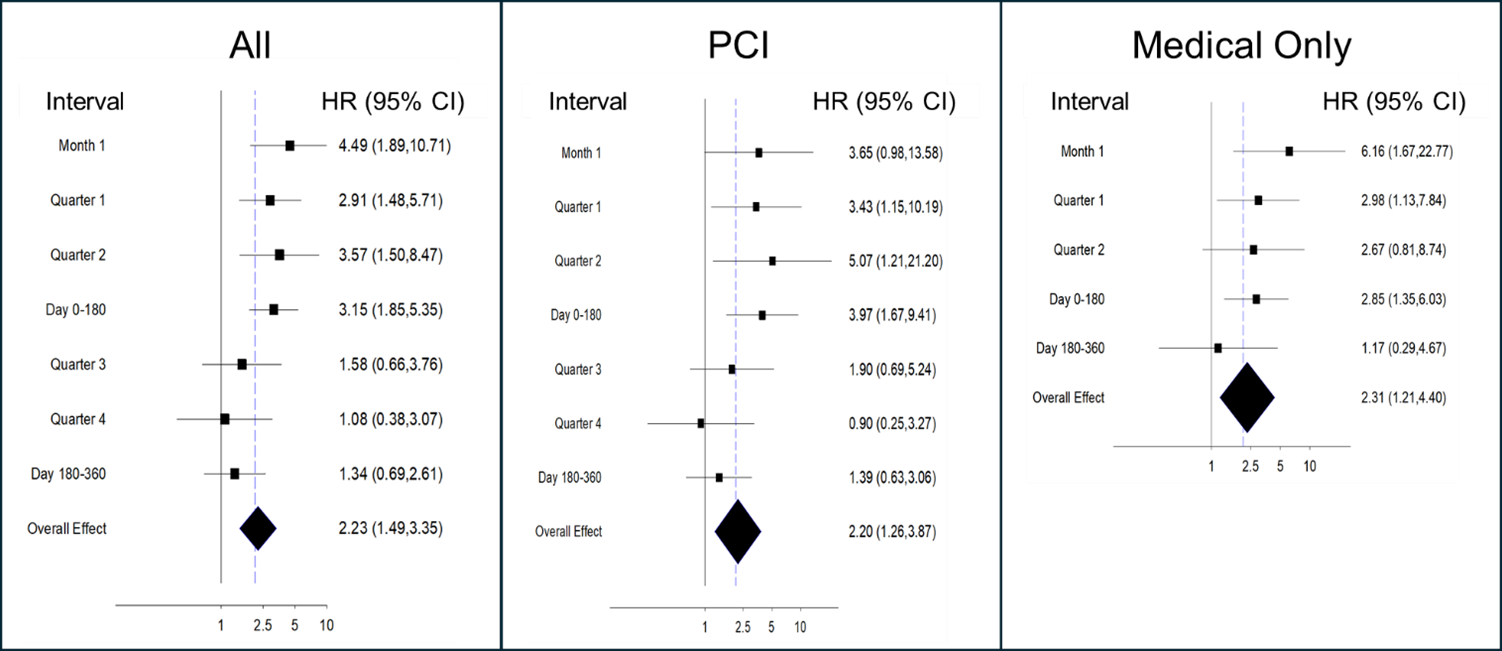
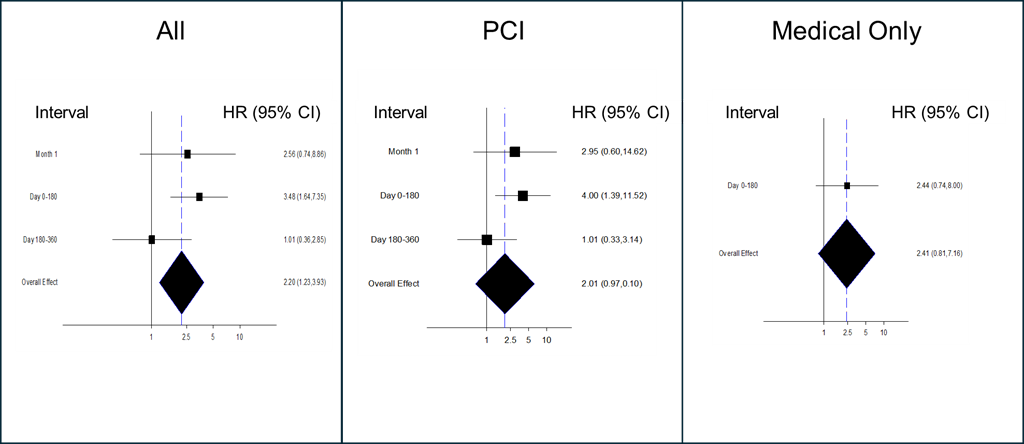
Analysis of the time to first event demonstrated that the pFCG test had the greatest prognostic power early after MI (Figures 1-3). The HR for the primary composite endpoint in all subjects (Figure 1) was greatest during the first month (3.84, P = .0009), and the HR for the first 6 months was 2.90 (P = .00005). Multivariate analysis demonstrated that the pFCG test independently predicted risk at 1 month (HR, 3.46; P = .003) and 6 months (HR, 2.57; P = .0005). Similar trends were apparent for the group of patients treated with PCI (6-month HR, 3.52; P = .002) and the group treated with medical therapy alone (6-month HR, 2.87; P = .006). Analysis of components of the primary endpoint, MI and death, (Figure 2) as well as MI alone, (Figure 3) showed similar trends. The HR for the combination of MI and death during the first month was 4.49 (P = .0007) and the HR for the first 6 months was 3.15 (P = .00002). For MI alone, the HR for the first 6 months was 3.48 (P = .001). The HR for BARC 2, 3, and 5 bleeding was 1.64 (P = .21; n = 26) through 6 months and 1.73 (P = .10; n = 38) through 12 months.
The prognostic implications of the pFCG test were assessed as a continuous variable with the primary endpoint. When a continuous analysis was performed by dividing the pFCG test result by 1000, the HR at 1 month was 1.54 (P = .015) and the HR at 6 months was 1.58 (P = .00003). When a continuous analysis was performed based on doubling of the pFCG test (base 2 logarithm), the prognostic implications of the pFCG test were demonstrated by an HR at 1 month of 2.49 (P = .004) and an HR at 6 months of 2.44 (P = .00002).
Discussion
Analysis of the prognostic implications of the pFCG test over time demonstrated that the prognostic power of the test was greatest earlier, when cardiovascular events were more common. The prognostic power was similar in the groups of patients treated with medical therapy alone or with PCI, and consistent with that seen in the entire cohort. Further, we observed similar trends with the primary composite endpoint as well as components, MI and death, and MI alone.
In contemporary practice, the impact of bleeding on outcomes5 has led to recommendations that clinicians should consider de-escalating therapy in patients who are at high risk of bleeding complications.6,7,15 Because bleeding and recurrent MI have a similar impact on the risk of death,5 clinical decision making must balance the risks of bleeding with those of recurrent MI. The results presented here support the potential value of the pFCG test to inform clinical decision making by providing a powerful tool capable of identifying patients at higher and lower risk of ischemic events early after MI.
The prognostic implications of the pFCG test were marked during the first 6 months after MI; however, the prognostic implications between 6 months and 12 months were substantially less than those seen earlier. While the incidence of primary events was less between 6 and 12 months, 40% of the total events during the first year after MI occurred during this interval. Additional research will be required to understand the nature of the decrease in prognostic power between 6 and 12 months after MI. Changes in the inflammatory milieu may lead to changes in megakaryocyte production of FcɣRIIa and, thereby, the pFCG test. Such a change could influence prognostic implications of the pFCG test over time. Serial pFCG testing after MI will be important to understand changes in platelet FcɣRIIa expression.
We have previously reported17 that patients treated with medical therapy alone had a greater prevalence of high-risk clinical features, were more commonly treated with anticoagulants, had lower use of more powerful antiplatelet agents, and had higher pFCG expression. Despite these patients experiencing a greater incidence of ischemic events, the prognostic implications of the pFCG test were similar in the medical treatment group compared with the PCI group. In this report, the prognostic power of the pFCG test was similarly greater in the group of patients treated with medical therapy alone compared with the PCI group.
Notably, patients treated with medical therapy alone had a greater prevalence of renal disease and were more likely to be treated with anticoagulants;17 both factors were associated with a greater risk of bleeding. Patients in the medical treatment group had a high risk of the primary endpoint (~24%) and a particularly high risk of death (~20%), likely reflecting the impact of comorbidities (which may have influenced the decision to pursue medical therapy alone) combined with MI. In this high-risk group, the pFCG test effectively identified patients at higher and lower risk of ischemic events. The results of the pFCG test could be a useful aid in risk-informed clinical decision making among these high-risk patients who are often at high risk of bleeding as well as ischemic events.
The pFCG test has been designed to be a reliable prognostic test that is performed on fixed platelets with the use of flow cytometry for excellent precision and accuracy.18 Results reflect expression of FcɣRIIa on the surface of approximately 10 000 platelets that can be quantified in approximately 1 minute. The pFCG test can be performed up to 5 days after fixation of platelets.
Limitations
Limitations of this study include (1) a single determination of the pFCG test shortly after MI that did not allow assessment of changes in this test over time; (2) the enrollment of a high-risk cohort rather than a cohort with wide range of clinical risk, which limits extrapolation of these results to cohorts with lower risk (although a previous study8 demonstrated similar prognostic implications in a lower risk cohort); and (3) the limitation of the number of clinical events (98) accrued, which is reflected in the size of the CIs and limits the assessment of predictive risk 6 to 12 months after MI. The results of this study support further research to understand the mechanisms responsible for the lower prognostic power 6 to 12 months after MI, as well as the design of interventional trials to use the pFCG test to guide individualized treatment.
Conclusions
The pFCG test is a powerful prognostic marker of ischemic risk, especially during the first 6 months after MI. The pFCG test stratifies patients at higher and lower risk of subsequent cardiovascular events, regardless of treatment strategy. The prognostic information provided by the pFCG test should be useful to clinicians as they balance risk of ischemic events with that of bleeding to define a treatment strategy. This test is not currently cleared by the Food and Drug Administration for clinical use. Future studies will be necessary to define specific treatment strategies based on the results of the pFCG test in combination with clinical risk assessment of ischemia and bleeding.
Affiliations and Disclosures
David J. Schneider, MD1; Sean R. McMahon, MD2; Dominick J. Angiolillo, MD, PhD3; Alexander C. Fanaroff, MD, MHS4; Homam Ibrahim, MD5; Patrick K. Hohl, DO6; Brett L. Wanamaker, MD7; Mark B. Effron, MD8; Peter M. DiBattiste, MD9; on behalf of the investigators
From the 1Department of Medicine, Cardiovascular Research Institute, The University of Vermont, Burlington, Vermont; 2Department of Medicine, Hartford Hospital, Hartford, Connecticut; 3Department of Medicine, Division of Cardiology, University of Florida, Jacksonville, Florida; 4Department of Medicine, University of Pennsylvania, Philadelphia, Pennsylvania; 5Adventist Healthcare White Oak, Silver Spring, Maryland; 6Division of Cardiovascular Medicine, Maine Medical Center, Portland, Maine; 7Division of Cardiovascular Medicine, University of Michigan, Ann Arbor, Michigan; 8John Ochsner Heart and Vascular Institute, University of Queensland Ochsner Clinical School, New Orleans, Louisiana; 9Prolocor, Inc, Philadelphia, Pennsylvania.
Acknowledgments: This study was supported by Prolocor, Inc. Expert statistical support was provided by Steven Snapinn, Seattle-Quilcene Biostatistics, LLC. The authors thank the investigators and coordinators at the participating sites. Sites and investigators included the University of Vermont Medical Center (David J. Schneider), Hartford Hospital (Sean R. McMahon), the University of Pennsylvania (Alexander C. Fanaroff), New York University (Claudia Serrano-Gomez), the Maine Medical Center (Patrick K. Hohl), the University of Florida (Dominick J. Angiolillo), Ascension St Vincent (Kevin M Ball), the University of Michigan (Brett L. Wanamaker), the John Ochsner Heart and Vascular Institute (Mark B. Effron), the Lankenau Medical Center (Timothy A Shapiro), St. Lukes Health System (Brian Nolan), Prairie Education and Research Cooperative (Abdalla Hassan), RWJ Barnabas Health (Marc Cohen), Ascension St. John (David Rodriguez), Ascension Sacred Heart (Rohit Amin), Sinai Center for Thrombosis Research (Paul A Gurbel), the University of California Los Angeles (William J French), Ascension St. Thomas (Timir Paul), Ascension Seton (Robert Shutt), Our Lady of Lourdes Memorial Hospital (Jerry Pudusseri), Ascension Via Christi Hospital (Wassim Shaheem), Ascension Macomb (Brilio Mojares).
Disclosures: Dr Schneider is a named inventor on patents (US 10,502,737; US 11,747,335 B2) that propose the use of FcγRIIa for assaying platelet reactivity and treatment selection, and is a co-founder of Prolocor, Inc. Dr Angiolillo has received consulting fees or honoraria from Abbott, Amgen, AstraZeneca, Bayer, Biosensors, Boehringer Ingelheim, Bristol-Myers Squibb, Chiesi, CSL-Behring, Daiichi-Sankyo, Eli Lilly, Faraday, Haemonetics, Janssen, Merck, Novartis, Novo Nordisk, PhaseBio, PLx Pharma, Pfizer, Sanofi, and Vectura, outside of the present work; his institution has received research grants from Amgen, AstraZeneca, Bayer, Biosensors, CeloNova, CSL Behring, Daiichi-Sankyo, Eisai, Eli Lilly and Company, Faraday, Gilead, Janssen, Matsutani Chemical Industry Co., Merck, Novartis, Osprey Medical, Renal Guard Solutions, and the Scott R. MacKenzie Foundation. Dr Wanamaker has received consulting fees from Asahi Intecc USA, Inc. Dr Effron has received a pension from, and has equity in Eli Lilly and Company. Dr DiBattiste is a co-founder of Prolocor, Inc. The remaining authors report no financial relationships or conflicts of interest regarding the content herein.
Funding: This study was supported by Prolocor, Inc.
Clinical trial registration information: https://clinicaltrials.gov/study/NCT05175261
Address for correspondence: David J. Schneider, MD, Cardiovascular Research Institute, The University of Vermont, 308 S. Park Drive, Colchester, VT 05446, USA. Email: David.Schneider@med.uvm.edu
References
- Yusuf S, Zhao F, Mehta SR, Chrolavicius S, Tognoni G, Fox KK; Clopidogrel in Unstable Angina to Prevent Recurrent Events Trial Investigators. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med. 2001;345(7):494-502. doi:10.1056/NEJMoa010746
- Wiviott SD, Braunwald E, McCabe CH, et al; TRITON-TIMI 38 Investigators. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357(20):2001-2015. doi:10.1056/NEJMoa0706482
- Wallentin L, Becker RC, Budaj A, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;36(11):1045-1057. doi:10.1056/NEJMoa0904327
- Watanabe H, Morimoto T, Natsuaki M, et al; STOPDAPT-2 ACS Investigators. Comparison of clopidogrel monotherapy after 1 to 2 months of dual antiplatelet therapy with 12 months of dual antiplatelet therapy in patients with acute coronary syndrome: the STOPDAPT-2 ACS randomized clinical trial. JAMA Cardiol. 2022;7(4):407-417. doi:10.1001/jamacardio.2021.5244
- Hara H, Takahashi K, Kogame N, et al. Impact of bleeding and myocardial infarction on mortality in all-comer patients undergoing percutaneous coronary intervention. Circ Cardiovasc Interv. 2020;13(9):e009177. doi:10.1161/CIRCINTERVENTIONS.120.009177
- Capodanno D, Bhatt DL, Gibson CM, et al. Bleeding avoidance strategies in percutaneous coronary intervention. Nat Rev Cardiol. 2022;19(2):117-132. doi:10.1038/s41569-021-00598-1
- Gorog DA, Ferreiro JL, Ahrens I, et al. De-escalation or abbreviation of dual antiplatelet therapy in acute coronary syndromes and percutaneous coronary intervention: a consensus statement from an international expert panel on coronary thrombosis. Nat Rev Cardiol. 2023;20(12):830-844. doi:10.1038/s41569-023-00901-2
- Schneider DJ, McMahon SR, Chava S, et al. FcγRIIa: a new cardiovascular risk marker. J Am Coll Cardiol. 2018;72(2):237-238. doi:10.1016/j.jacc.2018.04.046
- Schneider DJ, McMahon SR, Angiolillo DJ, et al. Platelet FcγRIIa as a marker of cardiovascular risk after myocardial infarction. J Am Coll Cardiol. 2024;84(18):1721-1729. doi:10.1016/j.jacc.2024.08.051
- Boylan B, Gao C, Rathore V, Gill JC, Newman DK, Newman PJ. Identification of FcgammaRIIa as the ITAM-bearing receptor mediating alphaIIbbeta3 outside-in integrin signaling in human platelets. Blood. 2008;112(7):2780-2786. doi:10.1182/blood-2008-02-142125
- Lova P, Paganini S, Sinigaglia F, Balduini C, Torti M. A Gi-dependent pathway is required for activation of the small GTPase Rap1B in human platelets. J Biol Chem. 2002;277(14):12009-12015. doi:10.1074/jbc.M111803200
- Serrano FA, El-Shahawy M, Solomon RJ, Sobel BE, Schneider DJ. Increased platelet expression of FcGammaRIIa and its potential impact on platelet reactivity in patients with end stage renal disease. Thromb J. 2007;5:7. doi:10.1186/1477-9560-5-7
- Zhi H, Rauova L, Hayes V, et al. Cooperative integrin/ITAM signaling in platelets enhances thrombus formation in vitro and in vivo. Blood. 2013;121(10):1858-1867. doi:10.1182/blood-2012-07-443325
- Schneider DJ, Taatjes-Sommer HS. Augmentation of megakaryocyte expression of FcgammaRIIa by interferon gamma. Arterioscler Thromb Vasc Biol. 2009;29(7):1138-1143. doi:10.1161/ATVBAHA.109.187567
- Matter MA, Paneni F, Libby P, et al. Inflammation in acute myocardial infarction: the good, the bad and the ugly. Eur Heart J. 2024;45(2):89-103. doi:10.1093/eurheartj/ehad486
- Mehran R, Rao SV, Bhatt DL, et al. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation. 2011;123(23):2736-2747. doi:10.1161/CIRCULATIONAHA.110.009449
- Schneider DJ, McMahon SR, Angiolillo DJ, et al; Investigators. Predictive value of platelet FcγRIIa in patients treated with PCI compared with medical therapy alone after myocardial infarction. Circ Cardiovasc Interv. 2025;18(4):e014939. doi:10.1161/CIRCINTERVENTIONS.124.014939
- Schneider DJ, Taatjes-Sommer HS, DiBattiste PM, et al. Assessing prognosis by quantifying FcγRIIa on fixed platelets. Bioanalysis. 2024;16(19-20):1025-1032. doi:10.1080/17576180.2024.2395706





















