Aortic Valve Calcification Alone Identifies Transcatheter Aortic Valve Replacement Benefit in Classical Low-Flow, Low-Gradient Aortic Stenosis
© 2025 HMP Global. All Rights Reserved.
Any views and opinions expressed are those of the author(s) and/or participants and do not necessarily reflect the views, policy, or position of the Journal of Invasive Cardiology or HMP Global, their employees, and affiliates.
J INVASIVE CARDIOL 2025. doi:10.25270/jic/25.00290. Epub September 25, 2025.
Abstract
Aortic valve calcification (AVC) is a guideline-endorsed diagnostic tool in low-flow, low-gradient (LFLG) aortic stenosis (AS), but its prognostic value across LFLG subtypes remains debated. In this retrospective cohort of 457 patients undergoing transcatheter aortic valve replacement (TAVR), severe AVC was associated with significantly fewer heart failure rehospitalizations in classical LFLG AS (adjusted hazard ratio [HR], 0.41; P = .023) but not in paradoxical LFLG AS (adjusted HR, 0.76; P = .422). AVC also correlated with true-severe AS when dobutamine stress echocardiography (DSE) was performed. These findings suggest that AVC alone can identify classical LFLG AS patients likely to benefit from TAVR, even when DSE results are unavailable or inconclusive.
Introduction
Low-flow, low-gradient (LFLG) aortic stenosis (AS) poses a unique diagnostic and therapeutic challenge because of discordant echocardiographic parameters and elevated post-transcatheter aortic valve replacement (TAVR) risk.1 The current standard for distinguishing true-severe from pseudo-severe AS involves dobutamine stress echocardiography (DSE); however, emerging data question DSE’s prognostic utility.2,3
Aortic valve calcification (AVC), quantified by gated computed tomography (CT), is guideline-endorsed as a diagnostic adjunct in LFLG AS, particularly when DSE is inconclusive.4,5 However, whether AVC alone provides useful diagnostic and prognostic information—especially for patients with unverified AS severity—remains less clear.6-8
Our research group previously reported that severe AVC was associated with a lower risk of heart failure (HF) rehospitalization after TAVR in a real-world cohort of LFLG AS patients in which DSE was often unavailable or inconclusive.9 In this study, we performed an additional sub-analysis to understand if the prognostic relevance of AVC differed by LFLG AS subtype: classical LFLG (cLFLG; left ventricular ejection fraction [LVEF] <50%) vs paradoxical LFLG AS (pLFLG; LVEF ≥50%).
Methods
Our study included 457 patients with LFLG AS (aortic valve area ≤1.0 cm², stroke volume index ≤35 mL/m², and mean gradient <40 mm Hg) who underwent TAVR at a single quaternary center between 2019 and 2021. The study was approved by the Mount Sinai Institutional Review Board with a waiver of informed consent due to its retrospective design.
AVC severity was assessed via Agatston score on multidetector CT using guideline-based sex-specific thresholds for severe AVC (≥2000 in men, ≥1300 in women).4,5 When available, AS severity was adjudicated using DSE.
Patients were stratified by LFLG subtype (cLFLG vs pLFLG) and by AVC severity (severe vs mild/moderate). The primary outcome was post-TAVR HF rehospitalization; all-cause mortality served as a secondary endpoint. Outcomes were evaluated using propensity-matched Cox regression models, adjusting for age, sex, coronary artery disease (CAD) requiring revascularization, chronic kidney disease, prior stroke, moderate-or-greater mitral regurgitation, atrial fibrillation, prior cardiac resynchronization therapy, New York Heart Association functional class, and right-sided cardiac damage (defined as stage 3 or 4 per the echocardiographic framework proposed by Nakase et al to capture pulmonary hypertension, tricuspid valve disease, and right ventricular dysfunction).10
Results
Baseline characteristics are summarized in the Table. For both mild/moderate AVC and severe AVC, the cLFLG AS groups had higher proportions of male patients and patients with a history of CAD, prior coronary artery bypass grafting, and percutaneous coronary intervention compared with the pLFLG AS groups.
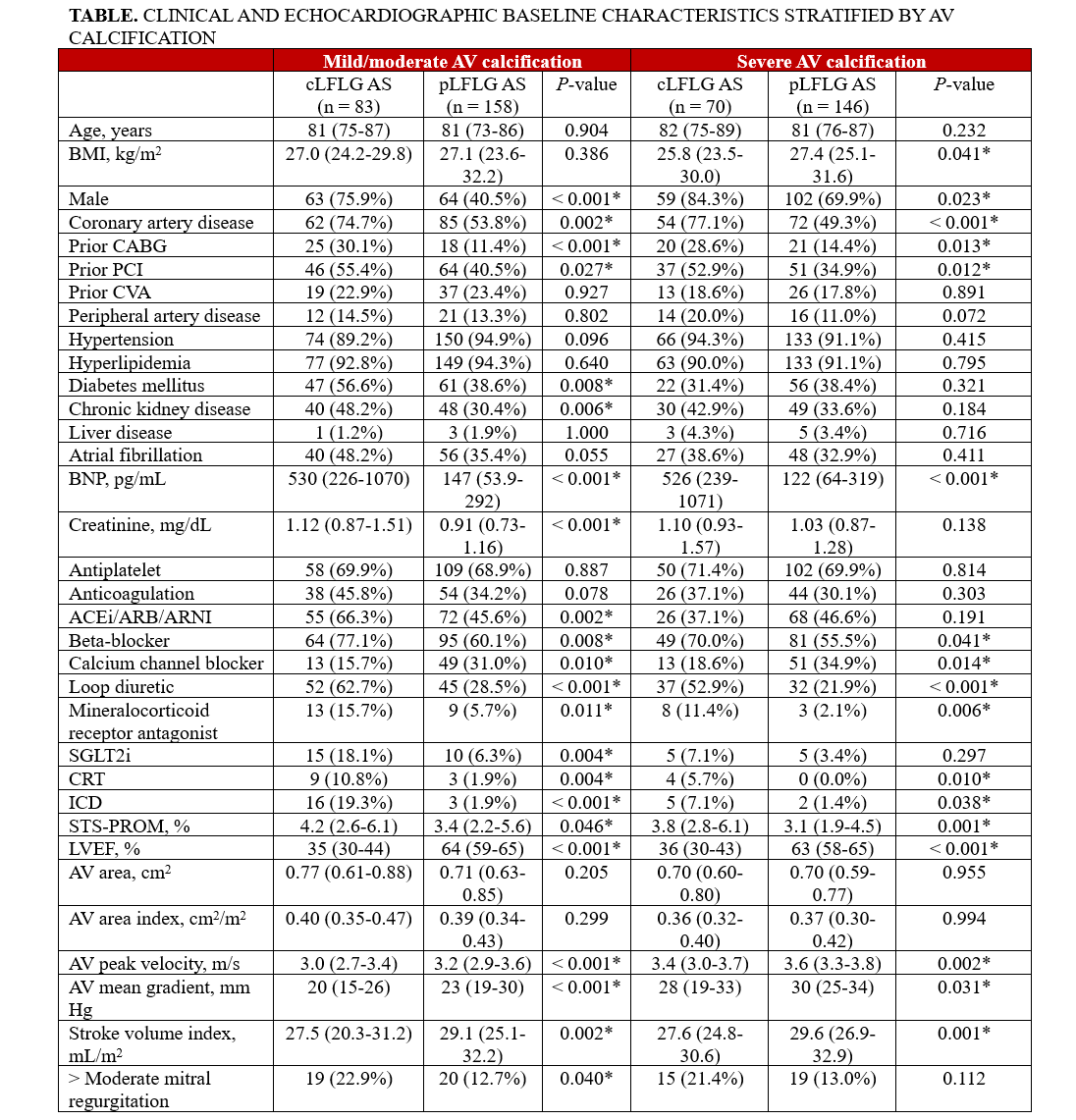
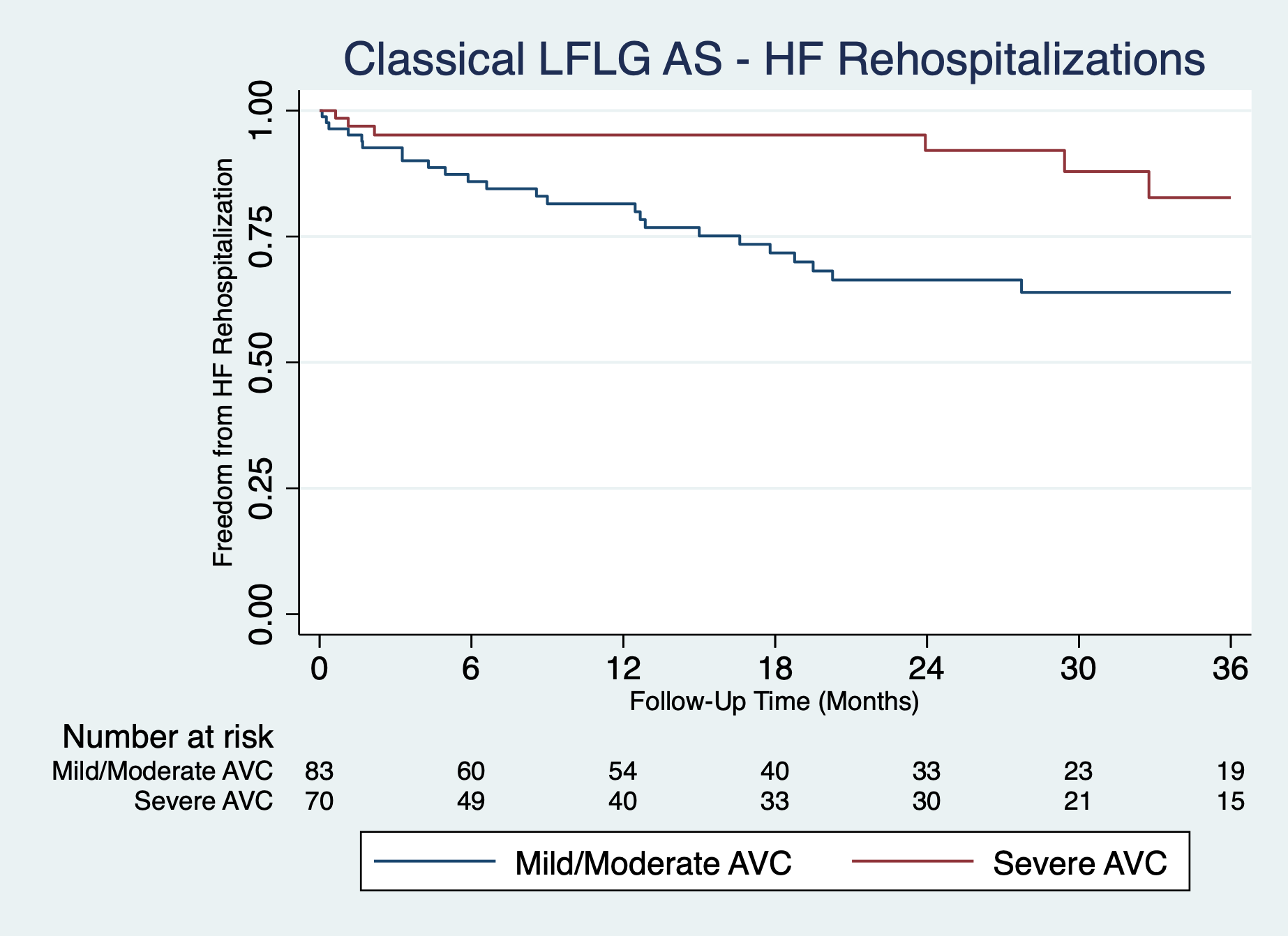
Among 457 patients, 216 (47.3%) had severe AVC. Propensity score matching in the cLFLG AS subgroup successfully matched 152 of 153 (99%) patients, achieving excellent covariate balance with all SDs less than 10%. In the propensity score-matched cLFLG AS subgroup, severe AVC was significantly associated with fewer HF hospitalizations (adjusted hazard ratio [HR], 0.41; 95% CI, 0.19-0.89; P = .023) (Figure). No such association was observed in the pLFLG AS group (adjusted HR, 0.76; 95% CI 0.39-1.49; P = .422). AVC severity was not associated with post-TAVR all-cause mortality in either cLFLG (adjusted HR, 1.14; 95% CI, 0.68-1.93; P = .615) or pLFLG (adjusted HR, 0.86; 95% CI 0.51-1.44; P = .569) AS groups.
Of the 153 patients with cLFLG AS, 35 (22.9%) underwent DSE to adjudicate AS severity. Among these 35 patients, verified true-severe AS was present in 9 of 10 (90%) patients with severe AVC, compared with only 9 of 25 (36%) patients with mild/moderate AVC.
Discussion
Our study revealed that the prognostic value of AVC differs by LFLG AS subtype. Unlike prior studies that focused primarily on AVC and mortality, our analysis uniquely demonstrates that severe AVC independently predicts reduced risk of HF rehospitalization following TAVR in patients with cLFLG AS.6-8 It is possible that the cardiac dysfunction of LFLG AS patients with severe AVC is driven largely by their valvulopathy—more so than those with LFLG AS and mild or moderate AVC—so treatment of that valvulopathy yields greater benefit. This selective prognostic value highlights the potential role of AVC in identifying patients with cLFLG AS who are more likely to derive meaningful clinical improvement after TAVR.
Importantly, the prognostic relevance of AVC persisted even without consistent use of DSE. Among the subset of cLFLG patients did underwent DSE, severe AVC was strongly associated with true-severe AS, while mild or moderate AVC was more often linked to pseudo-severe AS. These findings suggest that our survival analysis in the cLFLG group may reflect a natural separation between patients with true- vs pseudo-severe disease. However, within our real-world cohort—in which DSE was unavailable or inconclusive—AVC alone effectively identified patients more likely to benefit from TAVR. This has both clinical and financial implications: AVC may serve as a practical diagnostic and prognostic tool in cLFLG AS, potentially obviating the need for DSE, which is resource-intensive and often indeterminate.
In contrast, we found no association between AVC severity and post-TAVR outcomes in patients with pLFLG AS. This may reflect fundamental differences in underlying ventricular pathophysiology. In cLFLG AS, reduced EF often reflects a fixed systolic impairment, for which afterload reduction via TAVR can yield immediate hemodynamic and clinical benefit.11 In pLFLG AS, however, preserved systolic function masks underlying diastolic dysfunction where symptom improvement may depend more on postprocedural remodeling rather than on valve replacement alone. These mechanistic differences may limit the utility of AVC as a prognostic tool in pLFLG AS and underscore the need for distinct risk stratification strategies across LFLG subtypes.
Limitations
Several limitations of this study merit consideration. As a retrospective, nonrandomized analysis, our findings are subject to treatment selection bias, as only patients who were deemed suitable for and ultimately underwent TAVR were included. This inherently limits the generalizability of our results to the broader population of patients with LFLG AS, particularly those who are managed conservatively or considered ineligible for intervention. Additionally, the median follow-up period of approximately 2 years may have been insufficient to capture the full spectrum of long-term complications or delayed structural remodeling benefits associated with valve replacement in this frail patient population. Future prospective studies are warranted to validate the role of AVC-guided risk stratification for TAVR in LFLG AS.
Conclusions
AVC effectively identified classical LFLG AS patients who experienced the greatest clinical benefit from TAVR, even without DSE. These findings reinforce the role of AVC as a standalone tool to guide patient selection for TAVR.
Affiliations and Disclosures
Yash Prakash, MD1; Eric J. Kim, MD, MBE1; Ranbir Singh, MD2; Lakshay Chopra, MD3; Akarsh Sharma, MD1; Eileen Galvani, MD1; Thomas Hanlon, MD1; Ankita Naraparaju, MD1; Carlo Mannina, MD3; Oludamilola Akinmolayemi, MD, MPH2; Annapoorna S. Kini, MD2; Samin K. Sharma, MD2; Stamatios Lerakis, MD, PhD2
From the 1Samuel Bronfman Department of Medicine, Mount Sinai Hospital, New York, New York; 2Mount Sinai Fuster Heart Hospital, New York, New York; 3Department of Cardiology, Mount Sinai Morningside and West, New York, New York.
Dr Prakash and Dr Kim served as co-first authors.
Disclosures: The authors report no financial relationships or conflicts of interest regarding the content herein.
Artificial intelligence statement: During the preparation of this work, the authors used Claude AI (Anthropic) solely for assistance with statistical software coding, and ChatGPT (OpenAI) solely for language editing of the manuscript. All content and code generated with these tools were reviewed and edited by the authors, who take full responsibility for the accuracy and integrity of the final manuscript.
Address for correspondence: Stamatios Lerakis, MD, PhD, Icahn School of Medicine at Mount Sinai, 1 Gustave L. Levy Place, New York, NY 10029, USA. Email: stamatios.lerakis@mountsinai.org
References
- Clavel MA, Magne J, Pibarot P. Low-gradient aortic stenosis. Eur Heart J. 2016;37(34):2645-2657. doi:10.1093/eurheartj/ehw096
- Ribeiro HB, Lerakis S, Gilard M, et al. Transcatheter aortic valve replacement in patients with low-flow, low-gradient aortic stenosis. J Am Coll Cardiol. 2018;71(12):1297-1308. doi:10.1016/j.jacc.2018.01.054
- Maes F, Lerakis S, Ribeiro HB, et al. Outcomes from transcatheter aortic valve replacement in patients with low-flow, low-gradient aortic stenosis and left ventricular ejection fraction less than 30%: a substudy from the TOPAS-TAVI registry. JAMA Cardiol. 2019;4(1):64-70. doi:10.1001/jamacardio.2018.4320
- Otto CM, Nishimura RA, Bonow RO, et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease. J Am Coll Cardiol. 2021;77(4):e25-e197. doi:10.1016/j.jacc.2020.11.018
- Vahanian A, Beyersdorf F, Praz F, et al; ESC/EACTS Scientific Document Group. 2021 ESC/EACTS guidelines for the management of valvular heart disease: developed by the Task Force for the Management of Valvular Heart Disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Rev Esp Cardiol (Engl Ed). 2022;75(6):524-533. doi:10.1016/j.rec.2022.05.006
- Clavel MA, Pibarot P, Messika-Zeitoun D, et al. Impact of aortic valve calcification, as measured by MDCT, on survival in patients with aortic stenosis. J Am Coll Cardiol. 2014;64(12):1202-1213. doi:10.1016/j.jacc.2014.05.066
- Adrichem R, Hokken TW, Bouwmeester S, et al. Diagnostic value of aortic valve calcification levels in the assessment of low-gradient aortic stenosis. JACC Cardiovasc Imaging. 2024;17(8):847-860. doi:10.1016/j.jcmg.2024.03.014
- Cueff C, Serfaty JM, Cimadevilla C, et al. Measurement of aortic valve calcification using multislice computed tomography: correlation with haemodynamic severity of aortic stenosis and clinical implication for patients with low ejection fraction. Heart. 2011;97(9):721-726. doi:10.1136/hrt.2010.198853
- Singh R, Prakash Y, Chopra L, et al. Effects of aortic valve calcification on transcatheter aortic valve replacement for low-flow, low-gradient aortic stenosis. Cardiovasc Revasc Med. 2025;80:38-44. doi:10.1016/j.carrev.2025.01.005.
- Nakase M, Tomii D, Heg D, et al. Long-term impact of cardiac damage following transcatheter aortic valve replacement. JACC Cardiovasc Interv. 2024;17(8):992-1003. doi:10.1016/j.jcin.2024.02.011
- Garbi M, MacCarthy P, Shah AM, Chambers JB. Classical and paradoxical low-flow, low-gradient aortic stenosis: a heart failure perspective. Struct Heart. 2018;2(1):3-9. doi:10.1080/24748706.2017.1384876




















