Procedural and Long-Term Thromboembolic Outcomes After Left Atrial Appendage Closure: Comparison of Patients With Reduced and Preserved Left Ventricular Ejection Fraction
© 2025 HMP Global. All Rights Reserved.
Any views and opinions expressed are those of the author(s) and/or participants and do not necessarily reflect the views, policy, or position of the Journal of Invasive Cardiology or HMP Global, their employees, and affiliates.
Abstract
Objectives. To evaluate the impact of left ventricular ejection fraction (LVEF) on periprocedural complications and long-term thromboembolic events in patients with non-valvular atrial fibrillation (NVAF) treated with interventional left atrial appendage closure (LAAC).
Methods. In a retrospective single-center study, a total of 612 patients who underwent successful interventional LAAC were divided into 2 groups: 139 patients with reduced LVEF (< 50%) and 473 patients with preserved LVEF (≥ 50%). Baseline characteristics, in-hospital procedural complications, and long-term thromboembolic events were compared between the 2 groups.
Results. Patients with reduced LVEF were more likely to be female with a higher CHA2DS2-VA-Score (median 5 vs 4; P < .0001) and had higher rates of diabetes mellitus (54% vs. 40%; P = .003) and coronary/peripheral artery disease (68% vs 41%; P < .0001). There was no significant difference in procedure-related complications (major or minor bleeding [2.1% vs 4.2%; P = .44], access site complications [0% vs 4.2%; P = .08], cardiac tamponade [0.7% vs 0.6%; P = .91], transient ischemic attack (TIA) [1.4% vs 0.4%; P = .19], stroke [0% vs 0%], and in-hospital death [0% vs 0%]) between the 2 groups. Both groups had a similar median duration of long-term follow-up (20 vs 19 months, respectively; P = .15). During follow-up, there was no significant difference in the rates of TIA (2.2% vs 1.1%; P = .32), stroke (0.7% vs 1.9%; P = .33), or systemic thromboembolic events (0.7% vs 0.4%; P = .66) between the 2 groups.
Conclusions. In patients with reduced LVEF, the procedural safety of LAAC and the long-term rate of thromboembolic events were consistently low and comparable to patients with preserved LVEF.
Introduction
Atrial fibrillation (AF) is a common arrhythmia in clinical practice with potentially debilitating consequences because of its association with an increased risk of stroke and systemic embolism, resulting in significant morbidity, mortality, and healthcare costs.1 Recent guidelines for the management of AF recommend the CHA2DS2-VA score tool to predict the risk of systemic embolism in patients with non-valvular AF (NVAF). Prophylactic oral anticoagulation is recommended for patients with a score of 2 or higher.2 However, a significant proportion of patients with AF may be poor candidates for oral anticoagulation therapy (OAC) because of a high risk of bleeding or history of major bleeding.3 Therefore, interventional left atrial appendage closure (LAAC) has emerged as a viable alternative for these patients. Randomized trials have shown that LAAC is non-inferior in efficacy to oral anticoagulation with vitamin K antagonists and direct oral anticoagulants (DOACs), with an additional reduction in mortality.4,5 Two observational propensity score–matched trials comparing LAAC vs DOACs showed a net clinical benefit of LAAC compared with DOACs because of superior efficacy. In both studies, LAAC was associated with reduced mortality.6,7 Based on the increasing evidence for LAAC in prevention of thromboembolic events in AF patients, the European Society of Cardiology (ESC) AF Guidelines state that percutaneous LAAC may be considered for stroke prevention in patients with AF and contraindications for long-term OAC (Class IIb-recommendation, level of evidence C).2
AF and reduced left ventricular ejection fraction (LVEF) may coexist and may be the cause and/or consequence of each other in a vicious cycle. The 2 entities are linked by common risk factors such as age, hypertension, diabetes mellitus (DM), and structural heart disease, as well as hemodynamic, electrophysiological, and neurohormonal alterations.8
Interestingly, the coexistence of reduced LVEF in AF patients has been shown to increase the risk of stroke and systemic thromboembolic events, mainly because of impaired LA and LAA mechanical function, despite treatment with OAC.9 Furthermore, reduced LVEF is independently associated with a high prevalence of LA thrombus formation.10-12 This may raise the question of whether LAAC is less effective in reducing the overall risk of thromboembolic events in this patient population compared with patients with preserved LVEF.
Four recent studies have shown that reduced LVEF did not affect periprocedural complication rates and the occurrence of post-procedural thromboembolic events within 12 months after interventional LAAC.13-16 However, long-term follow-up data comparing clinical thromboembolic events in patients with reduced LVEF to those with preserved LVEF are sparse.
The present study aimed to evaluate the long-term efficacy of interventional LAAC in preventing systemic thromboembolic events in patients with NVAF with reduced LVEF. Furthermore, we assessed the influence of reduced LVEF on periprocedural complications after interventional LAAC.
Methods
Study population
This study includes patients with AF who underwent successful transcatheter LAAC at the Heart Center Leipzig between January 2003 and May 2018. Enrolled patients were divided into 2 groups according to their LVEF: Preserved LVEF (defined as EF ≥ 50%) vs reduced LVEF (defined as EF < 50%). Patients with reduced LVEF were further subdivided into patients with an LVEF of 40% or less and patients with LVEF of 41% to 49%.
Exclusion criteria were lack of documented pre-procedural LVEF, refusal to participate in the study, loss of contact, and unsuccessful LAAC. Device implantation was not possible in 14 patients because of unsuitable patient anatomy, and device embolization occurred in 4 patients (all devices were retrieved percutaneously). The Figure shows the details of the patient enrollment process.
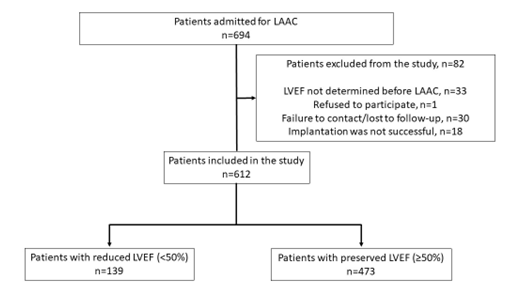
Prior to enrollment, all patients were provided with an informed consent form. Only patients who agreed to participate in the study were included. The study was conducted in accordance with the Declaration of Helsinki and approved by the local ethics committee. The authors confirm that informed consent was obtained from the patients for the study and intervention described in the manuscript and for its publication.
Data acquisition and assessment of in-hospital adverse events
Baseline and procedural characteristics as well as in-hospital adverse events were obtained from medical records. All patients were followed during hospitalization for periprocedural clinical adverse events, including intra-hospital death, stroke, transient ischemic attack (TIA), cardiac tamponade, and minor or major bleeding. The severity of periprocedural bleeding was defined according to the Bleeding Academic Research Consortium (BARC).17
Clinical follow-up
Clinical outcomes during follow-up were assessed based on hospital visits, scheduled follow-up visits, communication with outpatient physicians, and standardized telephone interview. Each enrolled patient was asked to complete a follow-up questionnaire to detect complications including TIA, stroke, or systemic embolization during follow-up. In the case of a history of stroke or thromboembolic events, the original medical reports were requested and reviewed by the study team. Post-procedural antithrombotic therapy was determined by indication and physician choice.
TEE was routinely performed at 45 days and 6 months to document adequate LAA closure without peri-device leak or device-related thrombus. Thereafter, post-procedural antithrombotic therapy was typically terminated.
Statistical analysis
Continuous variables are expressed as medians with IQR, and categorical variables are expressed as frequencies. Differences between continuous variables were evaluated using the Student’s t-test for normally distributed data and the Wilcoxon rank-sum test for non-normally distributed data. The chi-square test was used for categorical variables. A 2-tailed probability P-value of less than 0.05 was considered statistically significant. Logistic regression analyses were performed to identify predictors of long-term adverse clinical events (TIA, stroke, and systemic embolization). Odds ratios (OR) are presented with corresponding CIs calculated to the 95th percentile (95% CI). Statistical analyses were performed with SPSS software, Version 26 (IBM).
Results
Patient characteristics
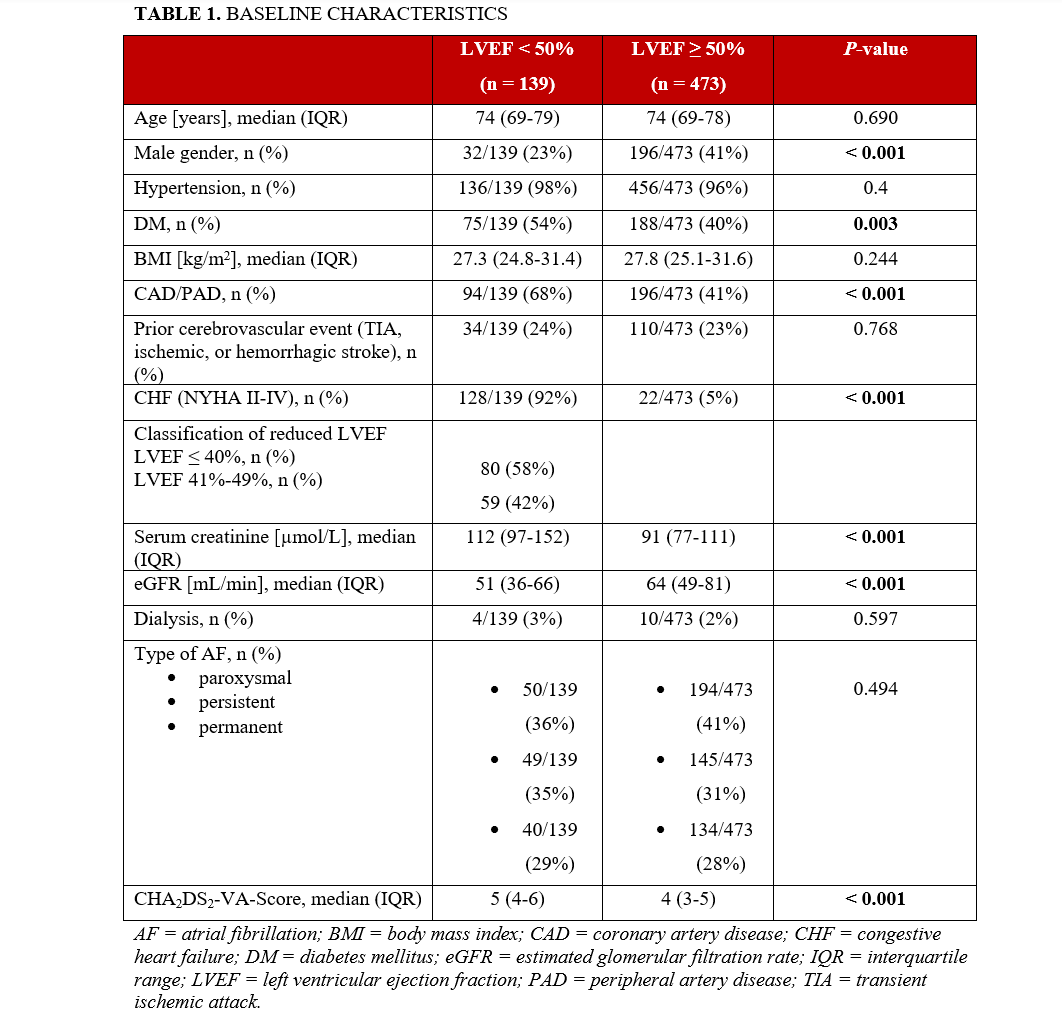
A total of 612 patients were enrolled. Of these, 139 (23%) patients had reduced LVEF (EF < 50%) and 473 (77%) had preserved LVEF. Of the patients with reduced LVEF, a total of 80 patients (58%) had an LVEF of 40% or less and 59 patients (42%) had an LVEF of 41% to 49%. Baseline characteristics are summarized in Table 1. Patients with reduced LVEF were more likely to be female and to have congestive heart failure (CHF), coronary artery disease (CAD)/peripheral artery disease (PAD), DM, and renal failure (P < .01). The median CHA2DS2-VA-Score was higher in this group (5 [IQR 4-6] vs 4 [IQR 3-5], P < .001).
All other baseline characteristics were balanced between the 2 groups.
In-hospital and long-term thromboembolic events
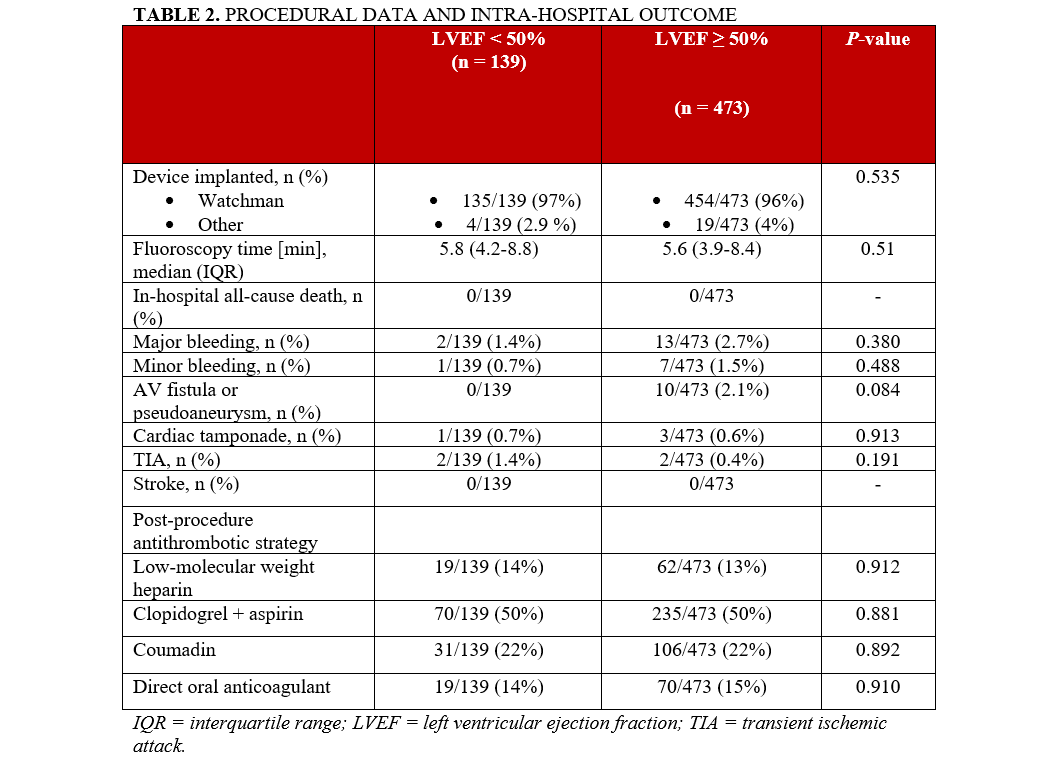
The WATCHMAN device (Boston Scientific) was used in the vast majority of the study population (n = 589, 96.3%). Periprocedural complications, defined as the occurrence of in-hospital death, stroke, TIA, cardiac tamponade, or minor or major bleeding, did not differ between the 2 groups (Table 2).
Post-procedural antithrombotic strategies for each group are summarized in Table 2.
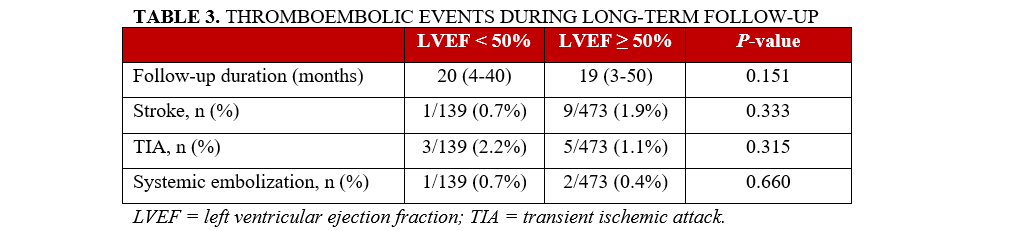
The median follow-up was 20 (IQR 4-40) months for the reduced LVEF group and 19 (IQR 3-50) months for the preserved LVEF group (P = .15). At least 1 follow-up visit was available for all of the patients. During follow-up, 14 patients (9.4%) in the reduced LVEF group and 35 patients (7.4%) in the preserved LVEF group died. The incidence of all-cause mortality was similar between the groups (P = .30).
The majority of patients discontinued anticoagulation or dual antiplatelet therapy within 1 year after LAAC (n = 128 [92%] in the reduced LVEF group vs n = 440 [93%] in the preserved LVEF group; P = .92).
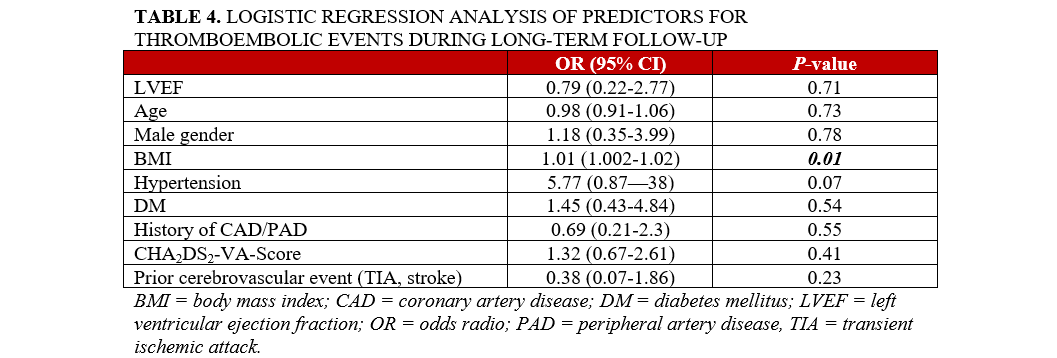
During long-term follow-up, the rates of stroke, TIA, and systemic embolism were similarly low in both groups (n = 16 [3.4%] vs n = 5 [3.6%]), as shown in Table 3. In multiple logistic regression, only body mass index (BMI) was identified as a predictor of thromboembolic clinical events during follow-up (OR = 1.01; 95% CI, 1.00-1.02; P = .01) (Table 4).
Discussion
The aim of the present analysis was to compare periprocedural complications and long-term thromboembolic events in patients with impaired vs preserved LVEF in NVAF undergoing interventional LAAC.
Several reports have suggested that the LAA is the dominant source of thrombus formation in patients with NVAF, and, therefore, its effective occlusion may reduce the risk of stroke.18 However, in certain subgroups, such as patients with reduced LVEF, the likelihood of non-LAA intracardiac thrombus is increased and, as a result, LAAC alone may not sufficiently eliminate the risk of thromboembolism.10-12 Assuming that the prevalence of non-LAA intracardiac thrombi is higher in patients with reduced LVEF than in patients with preserved LVEF, the lifetime risk of thromboembolism may remain higher despite LAAC. Therefore, long-term data are crucial. However, long-term data are scarce, and this study provides additional long-term data on the efficacy of LAAC in patients with reduced LVEF compared to those with preserved LVEF.
These data demonstrate that LAAC is associated with low rates of periprocedural complications. Notably, the rate of in-hospital and periprocedural complications in patients with reduced LVEF was low and comparable to that in patients with normal LVEF. It was notable that such low rates of in-hospital complications were achieved despite the higher burden of cardiovascular comorbidities such as DM, vascular disease, and CHF in the reduced-LVEF group compared to the group with preserved LVEF. These findings are consistent with a published subanalysis from the multicenter LAARGE (Left-Atrium-Appendage occluder Registry—Germany) registry. In this prospective non-randomized registry of patients undergoing LAAC, a total of 619 patients from 37 centers were divided into 3 groups (preserved LVEF, moderately reduced LVEF [35%-55%], and severely reduced LVEF [< 35%]). This study, which predominantly used the Amplatzer device (Abbott), showed that periprocedural complication rates in patients with reduced LVEF were not different from those in patients with preserved LVEF.13 Zhao et al also compared 2 groups (85 patients with CHF vs 316 patients without CHF) in terms of clinical outcome after transcatheter LAAC. Most patients in the CHF group (72%) had reduced LVEF (< 50%). The rates of intrahospital complications were comparable between the 2 groups.19
A study by Liu et al compared the safety and efficacy of LAAC in patients with heart failure. Patients with heart failure and reduced EF (LVEF ≤ 50%) were compared with patients with heart failure and preserved EF (LVEF > 50%). In this study, LAAC was found to be safe and effective in patients with AF and heart failure, regardless of the type of heart failure.16 It should be noted that patients with an LVEF of less than 30% were excluded from this study. A recent study by Saad et al compared the clinical outcomes of patients with CHF (defined as patients with LVEF < 50%, patients with preserved LVEF and diastolic dysfunction, or patients with right-sided heart failure) to patients without CHF. A total of 300 patients were enrolled (204 patients with CHF and 96 patients without CHF). The WATCHMAN device was implanted in approximately half of the patients (57% in the no-CHF group, 47% in the CHF group). The study showed that LAAC is equally safe in patients with reduced LVEF compared to patients without reduced LVEF.14
Das et al evaluated results from a large, nationwide US cohort of 34 385 patients with AF who underwent LAAC with the WATCHMAN device. The population was divided into 2 groups (patients with and without heart failure). The heart failure group included heart failure with reduced ejection fraction (HFrEF; n = 3780) and heart failure with preserved ejection fraction (HFpEF; n = 4750) patients. The 2 groups were compared for all‐cause in-hospital mortality and complications. The periprocedural complication rates were comparable between the 2 groups, but long-term outcomes were missing.15
Except for the study by Das et al, all of the above studies evaluated the occurrence of thromboembolic events (stroke, TIA, and systemic embolism) during follow-up.13,14,16,19 None of the studies found a difference in the rate of thromboembolic events between patients with reduced and preserved LVEF after interventional LAAC. In the study by Saad et al, the mean follow-ups were 176 days (CHF group) and 101 days (no-CHF group). In contrast to the study by Fastner et al, the rate of major adverse cardiac and cerebrovascular events (MACCE) during follow-up was significantly higher in the CHF group than in the no-CHF group. This difference was mainly due to increased mortality in the CHF group. This was explained by the older age and more frequent comorbidities in the CHF group. Cardiac mortality was similar in both groups. The study by Zhao et al also showed increased mortality in the CHF group. Over a mean of 2.2 years, the rates of all-cause death (P = .01) and cardiovascular death (P = .01) were significantly higher in the CHF group.19 In the current study, there was no statistically significant difference in all cause-mortality between the groups.
Five-year outcomes data from both the PREVAIL and PROTECT AF trials of the WATCHMAN device in patients with NVAF demonstrated excellent efficacy of interventional LAAC for long-term stroke prevention.5 However, neither clinical trial differentiated between patients with reduced and preserved LVEF. In addition, patients with a higher risk of intracardiac thrombi outside the LAA (especially those with LVEF < 30%) were excluded from these trials.
Our long-term follow-up data show low rates of TIA, stroke, and systemic embolization in patients with reduced LVEF and no difference from those with preserved LVEF in patients with AF undergoing LAAC. Logistic regression analysis showed that BMI was the only predictor of total thromboembolic events (TIA, stroke, and systemic embolization). These findings need to be confirmed in larger prospective studies.
Limitations
The retrospective, non-randomized design of the study and the moderate sample size from a single site with unequal group sizes are major limitations. In addition, the low event rates may have limited the statistical power to detect significant differences between groups.
Control transesophageal echocardiogram performed more than 6 months post-procedure was only done in the case of clinical events (TIA, stroke, or systemic embolism). Therefore, subclinical device-related thrombi may have remained undetected. Adverse events during follow-up were partially self-reported, which might have underestimated the rate of detected thromboembolic events.
Finally, the study reflects the experience of a high-volume center using primarily the earlier generation of the WATCHMAN device. Therefore, these results may not be generalizable to the newer, redesigned WATCHMAN FLX device or other devices for LAAC, or to all centers performing LAAC.
Conclusions
This single-center study provides additional evidence of the low rate of short- and long-term thromboembolic events with percutaneous LAAC, regardless of LVEF. Larger randomized prospective studies are needed to confirm our findings.
Affiliations and Disclosures
Nicolas Majunke, MD; Hamza El Hadi, MD, PhD; Steffen Desch, MD; Tobias Kister, MD; Maria Buske, MD; Natalie Fischer, MD; Katharina Kirsch, MD; Janine Pöss, MD; Holger Thiele, MD; Marcus Sandri, MD
From the Heart Center Leipzig, University of Leipzig, Department of Internal Medicine/Cardiology, Leipzig, Germany.
The abstract has been previously published (Clin Res Cardiol. 2023;112:1005. doi:10.1007/s00392-023-02180-w)
Disclosures: The authors report no financial relationships or conflicts of interest regarding the content herein.
Address for correspondence: Nicolas Majunke, MD, Heart Center Leipzig, University of Leipzig, Department of Internal Medicine/Cardiology, Strümpellstr. 39, D-04289 Leipzig, Germany. Email: nicolas.majunke@medizin.uni-leipzig.de
References
1. Essa H, Hill AM, Lip GYH. Atrial fibrillation and stroke. Card Electrophysiol Clin. 2021;13(1):243-255. doi:10.1016/j.ccep.2020.11.003
2. Van Gelder IC, Rienstra M, Bunting KV, et al; ESC Scientific Document Group. 2024 ESC Guidelines for the management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2024;45(36):3314-3414. doi:10.1093/eurheartj/ehae176
3. Seelig J, Pisters R, Hemels ME, Huisman MV, Ten Cate H, Alings M. When to withhold oral anticoagulation in atrial fibrillation - an overview of frequent clinical discussion topics. Vasc Health Risk Manag. 2019;15:399-408. doi:10.2147/VHRM.S187656
4. Osmancik P, Herman D, Neuzil P, et al. PRAGUE-17 Trial Investigators. 4-year outcomes after left atrial appendage closure versus nonwarfarin oral anticoagulation for atrial fibrillation. J Am Coll Cardiol. 2022;79:1-14. doi:10.1016/j.jacc.2021.10.023
5. Reddy VY, Doshi SK, Kar S, et al; PREVAIL and PROTECT AF Investigators. 5-Year outcomes after left atrial appendage closure: from the PREVAIL and PROTECT AF trials. J Am Coll Cardiol. 2017;70(24):2964-2975. doi:10.1016/j.jacc.2017.10.021
6. Nielsen-Kudsk JE, Korsholm K, Damgaard D, et al. Clinical outcomes associated with left atrial appendage occlusion versus direct oral anticoagulation in atrial fibrillation. JACC Cardiovasc Interv. 2021;14(1):69-78. doi:10.1016/j.jcin.2020.09.051
7. Gloekler S, Fürholz M, de Marchi S, et al. Left atrial appendage closure versus medical therapy in patients with atrial fibrillation: the APPLY study. EuroIntervention. 2020;16(9):e767-774. doi:10.4244/EIJ-D-20-00201
8. Carlisle MA, Fudim M, DeVore AD, Piccini JP. Heart failure and atrial fibrillation, like fire and fury. JACC Heart Fail. 2019;7(6):447-456. doi:10.1016/j.jchf.2019.03.005
9. Ellis CR, Kanagasundram AN. Atrial fibrillation in heart failure: left atrial appendage management. Cardiol Clin. 2019;37(2):241-249. doi:10.1016/j.ccl.2019.01.009
10. Wybraniec MT, Mizia-Szubryt M, Cichoń M, et al. Heart failure and the risk of left atrial thrombus formation in patients with atrial fibrillation or atrial flutter. ESC Heart Fail. 2022;9(6):4064-4076. doi:10.1002/ehf2.14105
11. Angebrandt Belošević P, Šmalcelj A, Kos N, Kordić K, Golubić K. Left ventricular ejection fraction can predict atrial thrombosis even in non-high-risk individuals with atrial fibrillation. J Clin Med. 2022;11(14):3965. doi:10.3390/jcm11143965
12. Mahajan R, Brooks AG, Sullivan T, et al. Importance of the underlying substrate in determining thrombus location in atrial fibrillation: implications for left atrial appendage closure. Heart. 2012;98(15):1120-1126. doi:10.1136/heartjnl-2012-301799
13. Fastner C, Brachmann J, Lewalter T, et al. Left atrial appendage closure in patients with a reduced left ventricular ejection fraction: results from the multicenter German LAARGE registry. Clin Res Cardiol. 2020;109(11):1333-1341. doi:10.1007/s00392-020-01627-8
14. Saad M, Osman M, Hasan-Ali H, et al. Atrial appendage closure in patients with heart failure and atrial fibrillation: industry-independent single-centre study. ESC Heart Fail. 2022;9(1):648-655. doi:10.1002/ehf2.13698
15. Das S, Lorente-Ros M, Wu L, Mehta D, Suri R. Safety of left atrial appendage closure in heart failure patients. J Cardiovasc Electrophysiol. 2022;33(12):2578-2584. doi:10.1111/jce.15682
16. Liu L, Yan W, Xu X, et al. Safety and effectiveness of left atrial appendage closure in atrial fibrillation patients with different types of heart failure. BMC Cardiovasc Disord. 2024;24(1):430. doi:10.1186/s12872-024-04094-5
17. Mehran R, Rao SV, Bhatt DL, et al. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation. 2011;123(23):2736-2747. doi:10.1161/CIRCULATIONAHA.110.009449
18. Safavi-Naeini P, Rasekh A. Closure of left atrial appendage to prevent stroke: devices and status. Tex Heart Inst J. 2018;45(3):172-174. doi:10.14503/THIJ-18-6693
19. Zhao M, Hou CR, Bai J, et al. Effect of congestive heart failure on safety and efficacy of left atrial appendage closure in patients with non-valvular atrial fibrillation. Expert Rev Med Devices. 2022;19(10):805-814. doi:10.1080/17434440.2022.2141112





















