Percutaneous Left Atrial Appendage Occlusion in Heart Failure: A Nationwide Readmission Database Analysis
© 2025 HMP Global. All Rights Reserved.
Any views and opinions expressed are those of the author(s) and/or participants and do not necessarily reflect the views, policy, or position of the Journal of Invasive Cardiology or HMP Global, their employees, and affiliates.
Abstract
Objectives. Left atrial appendage occlusion (LAAO) has emerged as an alternative to anticoagulation for reducing stroke risk in patients with atrial fibrillation (AF). However, patients with heart failure (HF) may have an enhanced risk of stroke and elevated risk for this procedure. To further investigate this population, the authors present a retrospective analysis on LAAO in patients with HF.
Methods. The authors performed a retrospective review of all hospitalizations for LAAO using the National Readmissions Database between September 2015 and November 2019. From these, patients with ICD-10 codes for HF were identified. Propensity matched (PSM) analysis was used to compare matched samples of patients with and without HF. Outcomes assessed included all-cause mortality, stroke, major bleeding, pericardial effusion, tamponade, and acute kidney injury (AKI).
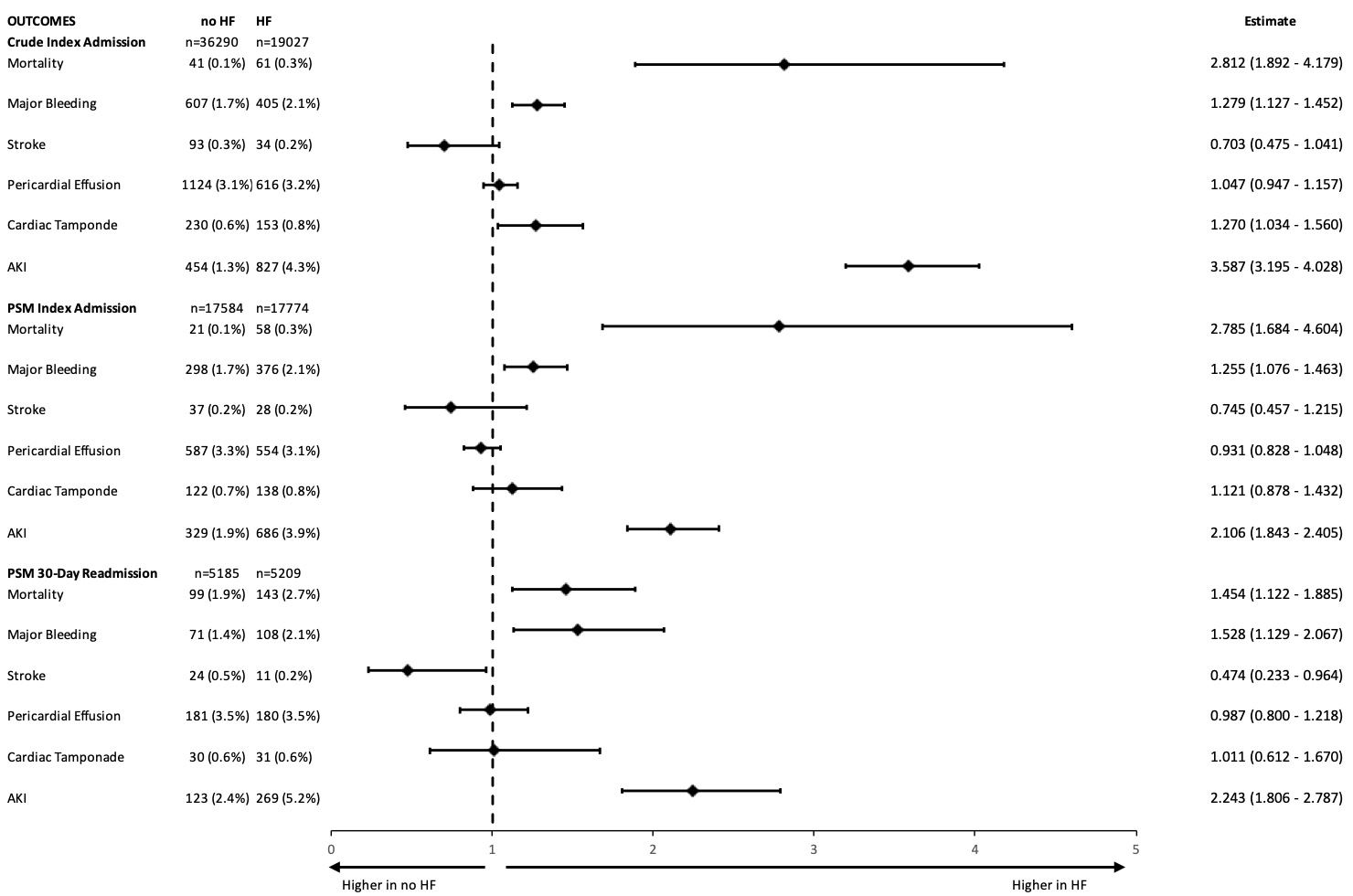
Results. After PSM, HF was associated with higher odds of mortality (odds ratio [OR] 2.79 [1.68-4.60]), major bleeding (OR 1.26 [1.08-1.46]), and AKI (OR 2.11 [1.84-2.41]) at index admission. Mortality (OR 1.45 [1.12-1.89]), major bleeding (OR 1.53 [1.13-2.07]), and AKI (OR 2.24 [1.81-2.79]) were also significantly higher at 30-day readmission. There was no significant increase in pericardial effusion, tamponade, or stroke after PSM at index admission or 30-day readmission.
Conclusions. The findings suggest LAAO use in HF is not associated with an increased risk of pericardial effusion, tamponade, and stroke in the periprocedural period when compared with those without HF. Mortality, major bleeding, and AKI were found to be modestly higher in patients with HF. Further investigation is warranted to evaluate the long-term risk of stroke in patients with HF with LAAO.
Introduction
The prevalence of atrial fibrillation (AF) in heart failure (HF) has been reported to be between 10% and 50%, with worsening HF correlating with a higher prevalence of AF.1-3 HF may lead to elevated left atrial filling pressures, activation of the neurohormonal cascade, and atrial remodeling, therefore predisposing patients to AF.4 Conversely, AF may facilitate the development or progression of HF through tachycardia-mediated cardiomyopathy. While it is commonly understood that AF increases the risk of stroke, studies have shown an elevated risk of stroke with HF and AF compared with AF alone, with HF conferring a relative risk of 1.4 to 3.1.5,6
Oral anticoagulation (OAC) is commonly used for stroke prevention in AF. While these agents have been shown to be effective in reducing stroke risk, there are many barriers to their use, including an increased bleeding risk, poor patient compliance, and high long-term cost.7 Percutaneous left atrial appendage occlusion (LAAO) is now considered a non-pharmacologic alternative to OAC in reducing stroke risk in non-valvular AF patients, as shown by the pivotal PREVAIL and PROTECT AF trials, though only 23.2% to 27.0% of participants had HF and patients with New York Heart Association (NYHA) class IV HF were excluded.8,9 As such, further investigation is warranted to evaluate the safety and efficacy of LAAO in the HF population. We present a retrospective, national registry-based comparison of periprocedural outcomes of LAAO in patients with and without HF.
Methods
Data source
Data acquisition was carried out using the Nationwide Readmissions Database (NRD), which is part of the Healthcare Cost and Utilization Project (HCUP). The NRD was established through a federal-state-industry partnership and is overseen by the Agency for Healthcare Research and Quality. It is an all-payer database that includes over 15 million discharge records from 22 US states. This unweighted data represents 49% of all US hospitalizations and covers 51% of the US population. To estimate national figures, the data from the NRD is weighted according to the city and hospital of origin. The NRD also includes unique identification codes that allow patients to be tracked over an 11-month period, enabling the capture of readmissions. All data are anonymized, so institutional review board (IRB) approval is not required.
Study design and population
The International Classification of Diseases-10th Revision-Clinical Modification (ICD-10-CM) codes for all hospitalizations for LAAO were identified between September 2015 and November 2019. Of these, patients with codes for acute and chronic, and systolic and diastolic HF were identified. Unfortunately, left ventricular ejection fraction (LVEF) and the presence of right ventricular dysfunction was not available to us, leading us to combine various forms of HF in our analysis. Because data are annualized, December hospitalizations from each year were excluded to allow for 30-day outcomes for each index admission. The LAAO of patients with HF were compared with a matched sample of patients undergoing LAAO without an ICD diagnosis of HF.
Outcomes
Analyzed outcomes included all-cause mortality, stroke, major bleeding, pericardial effusion, cardiac tamponade, and acute kidney injury (AKI). Outcomes were examined at index hospitalization and at 30-day readmission. The mean length of index hospitalization, the adjusted cost of index hospitalization, and the rate of 30-day rehospitalization over 11 months were also assessed. To evaluate relative risk reduction in stroke, all patients were assigned a CHA2DS2-VASc (C2V) score and, subsequently, a monthly risk of stroke was obtained for each patient by dividing the annual risk by 12.
Statistics
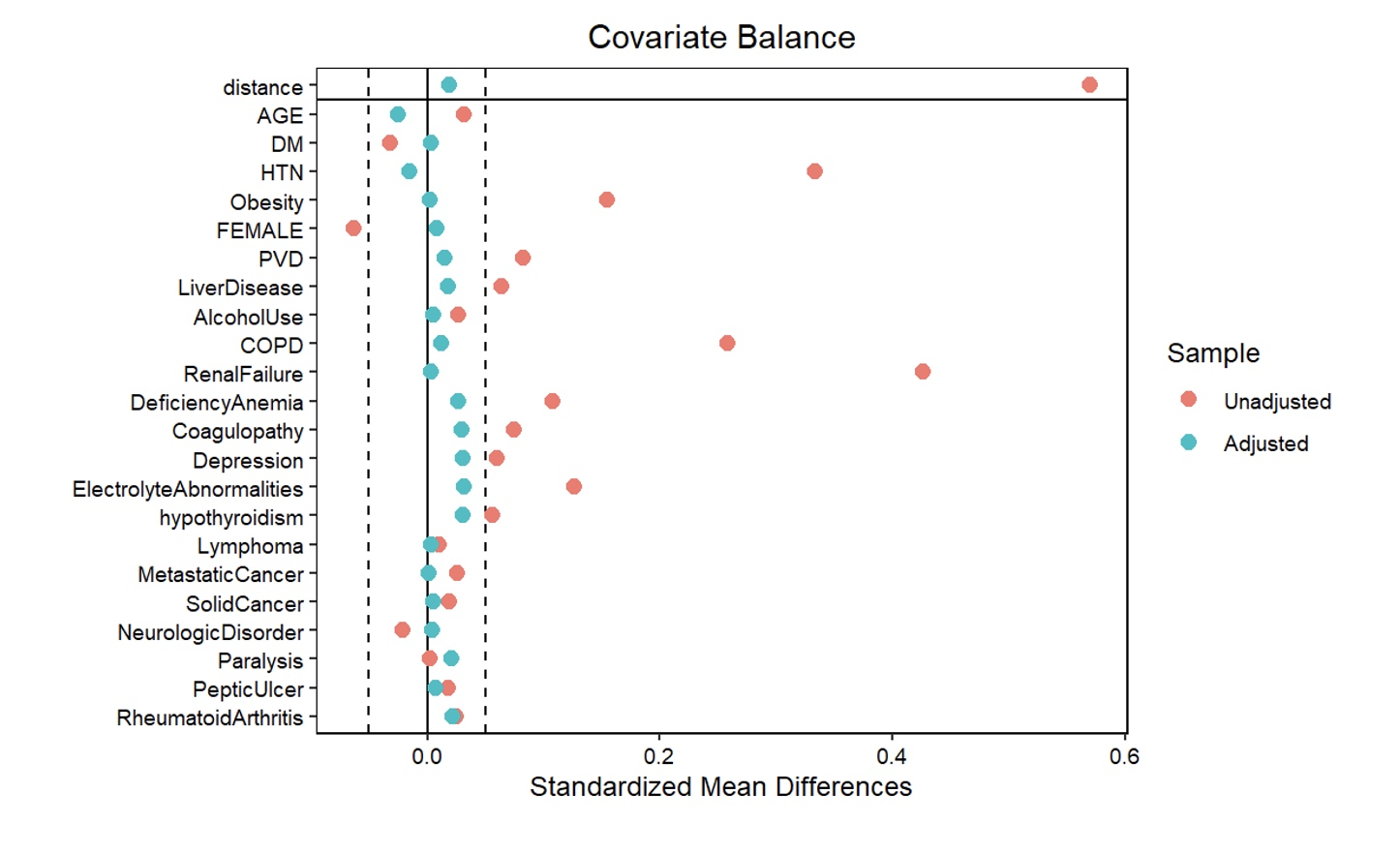
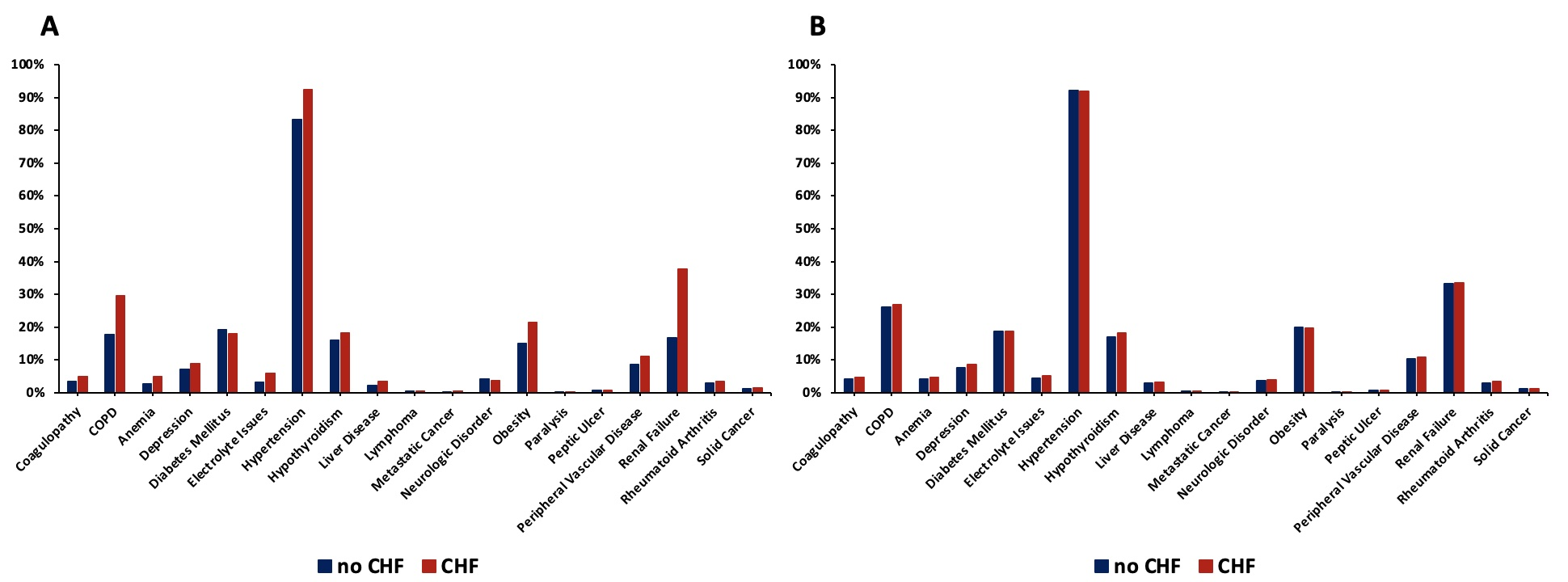
Clinical characteristics, comorbidities, and outcomes for each cohort (HF vs no HF) were presented as proportions for categorical data and as mean with SD for numerical variables. The unadjusted odds ratios (OR) with a 95% CI were computed using the Cochran-Mantel-Haenszel test. For adjusted ORs, 2 propensity score-matched (PSM) analyses were obtained: one for the index hospitalization at the time of LAAO and the other at 30-day readmission. A one-to-many near neighbor PSM strategy with an allowable threshold of 0.1 absolute standardized mean difference (SMD) and 0.05 Kolmogorov-Smirnov Statistic (KSS) was implemented without replacement. The variables used for propensity matching included age, sex, and major comorbidities, including coagulopathy, chronic obstructive pulmonary disease (COPD), anemia, depression, diabetes mellitus (DM), electrolyte abnormalities, hypertension, hypothyroidism, liver disease, lymphoma, metastatic cancer, neurologic disorder, obesity, paralysis, peptic ulcer, peripheral vascular disease (PVD), renal failure, rheumatoid arthritis, and solid cancer. The estimates of comorbidities were obtained using the proposed coding scheme as described previously.10 A comparison of comorbidities for the original cohort and the PSM-matched subcohort for the index hospitalization is provided in Figure 1.
For normally distributed data, the Analysis of Variance (ANOVA) test was used. To estimate the hospital cost, the NRD provided a ‘cost-to-charge ratio’ variable, which was multiplied by the total inpatient charges and was then adjusted for inflation until January 2020 using the Bureau of Labor Statistics Consumer Price Index (adjusted cost). A P-value of less than 0.05 was considered significant. All analyses were performed using R 3.02 (R Foundation for Statistical Computing) and SPSS 27 (IBM).
Results
Case selection
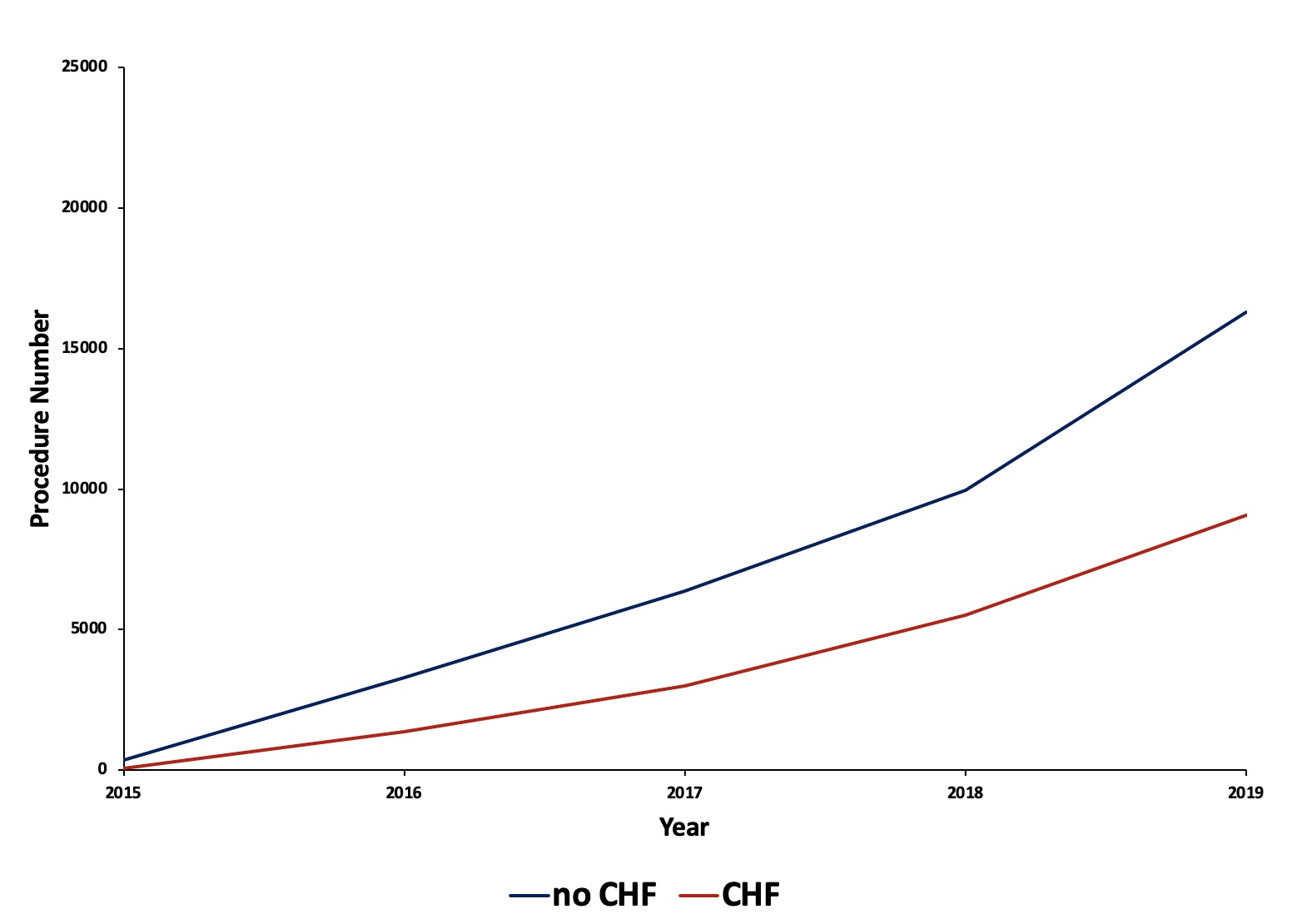
From September 2015 to November 2019, and after excluding December hospitalizations, 55 317 LAAO hospitalizations were identified. Out of these, 36 290 patients without a history of HF underwent LAAO, and 19 027 with a history of HF underwent LAAO. The trend of LAAO procedures per year for patients with and without HF is provided in Figure 2.
Patient characteristics
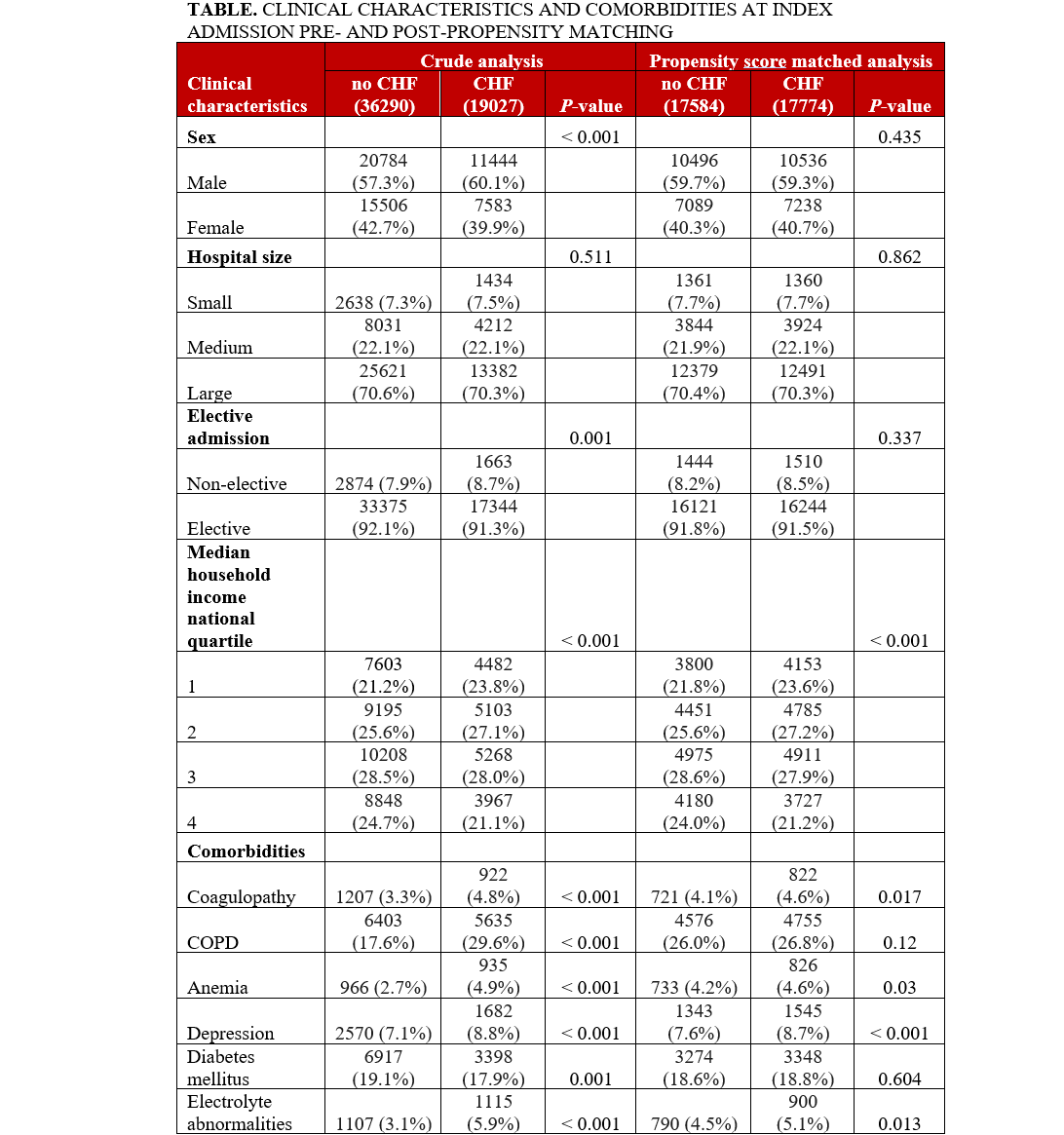
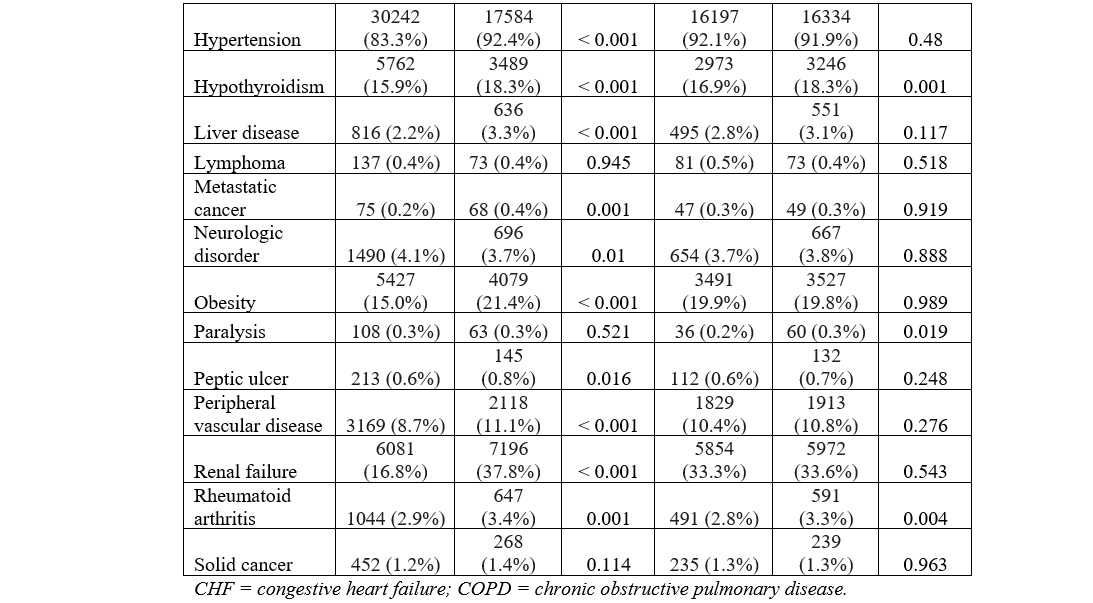
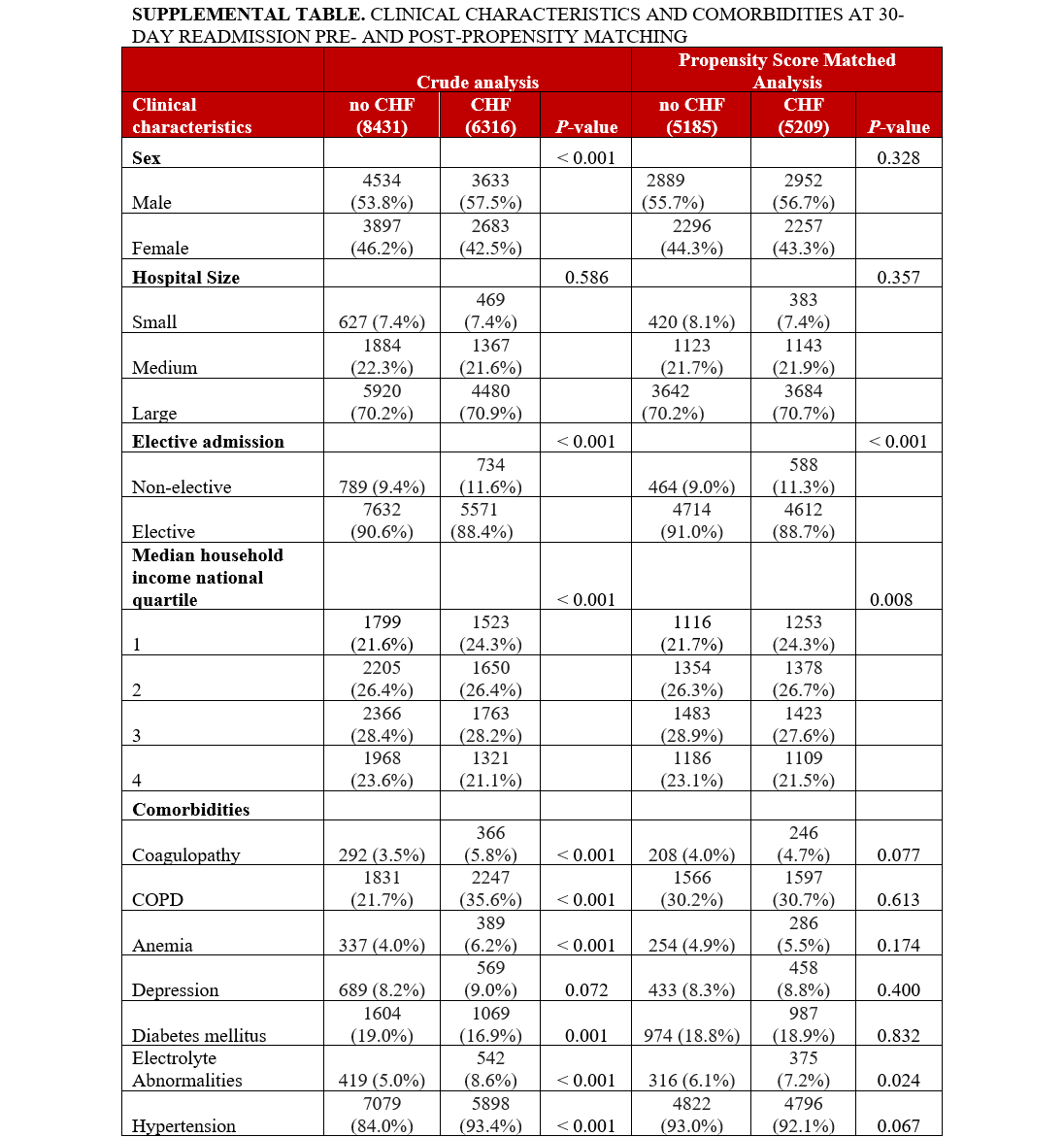
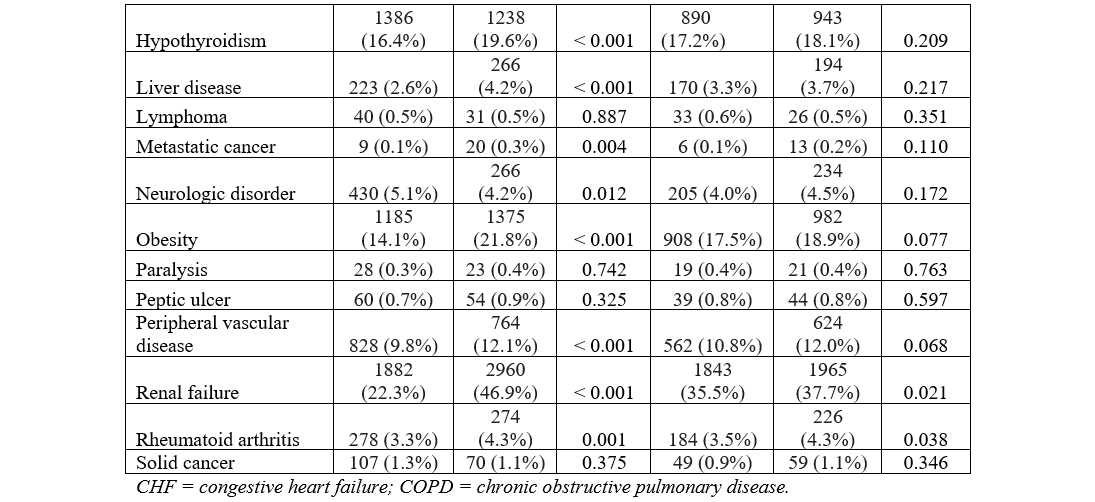
The baseline characteristics and comorbidities for the total population are provided in the Table. The mean age was 76.2 ± 7.9 years (HF: 76.3 ± 8.1, no HF: 76.1 ± 7.8, P = .001). Crude analysis revealed a significantly higher proportion of male patients in the HF group vs the no-HF group. Hospital size was not differently distributed across groups. The HF group had significantly higher non-elective admissions. A higher proportion of patients with HF were in the lowest median household income quartile compared with those without HF. Comorbidities of coagulopathy, COPD, anemia, depression, electrolyte abnormalities, hypertension, hypothyroidism, liver disease, metastatic cancer, obesity, peptic ulcer, PVD, renal failure, and rheumatoid arthritis were significantly higher in the HF group, as shown in Figure 3A. The prevalence of DM and neurologic disorders was higher in the no-HF group. Patient characteristics and comorbidities at 30-day readmission before and after PSM can be found in the Supplemental Table.
Outcomes with crude analysis
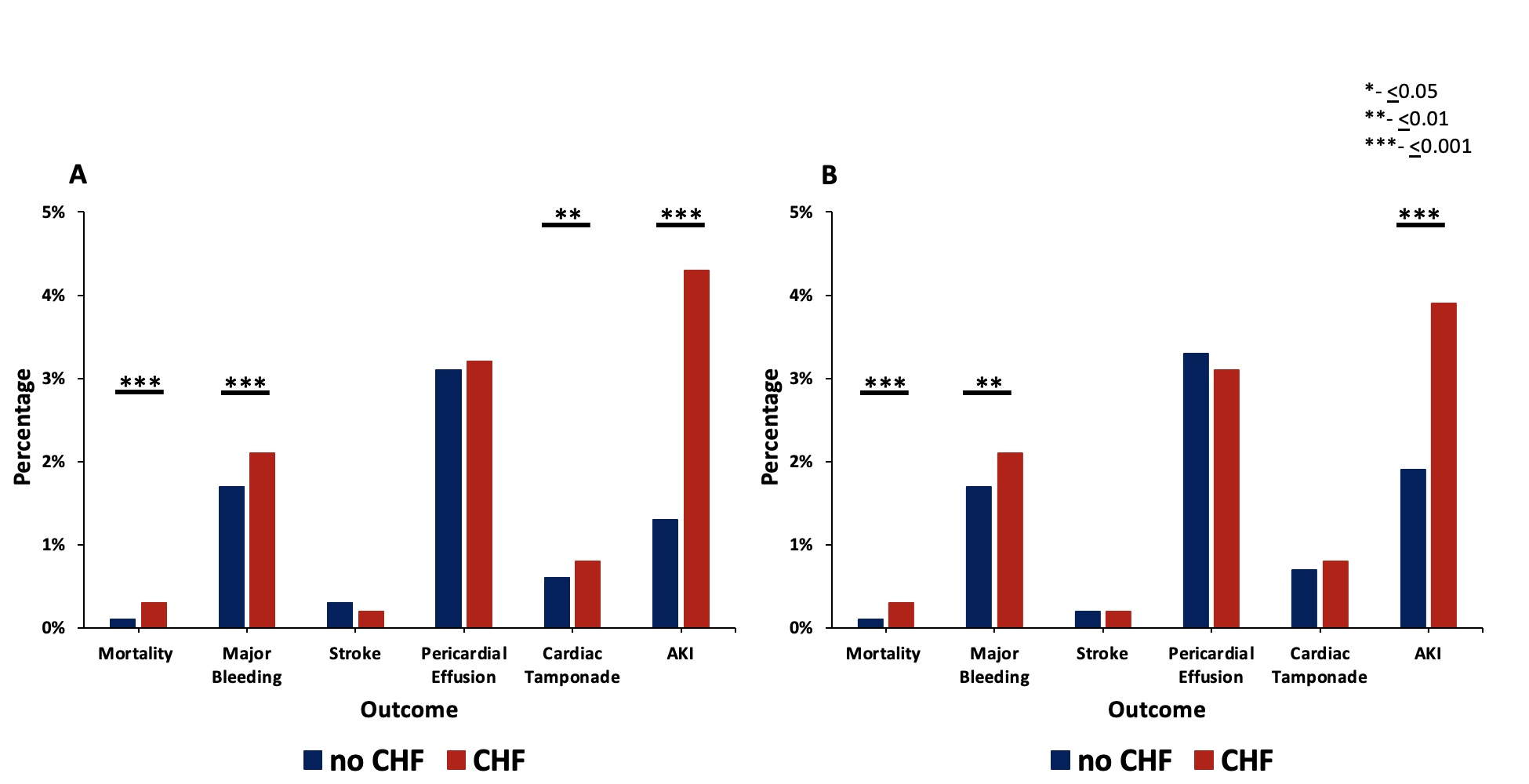
The unadjusted odds of mortality, major bleeding, cardiac tamponade, and AKI were all significantly higher in the HF cohort at index admission, and all but cardiac tamponade were again significantly higher in the HF cohort at 30-day readmission (Figure 4, Supplemental Figure 1A) Stroke and pericardial effusion were not significantly different between the 2 groups at index admission or at 30-day readmission. The mean length of stay of the index hospitalization was greater in the HF group compared with the no-HF group (1.73 ± 3.20 days vs 1.29 ± 1.81 days, P < .001). The mean cost of index hospitalization was also greater for the HF group compared with the no-HF group ($27 258 ± $14 169 vs $25 575 ± $11 455, P < .001). The mean C2V score for patients with HF was 4.52 ± 1.17, while the mean C2V score for patients without HF was 3.26 ± 1.15 (P < .001).
Outcomes with propensity matching for index admission
PSM analysis yielded a subcohort of 35 358 patients (17 774 with HF and 17 584 without HF). Baseline characteristics were similar including age (76.31 vs 76.47, P = .071), gender (59.3% vs 59.7% male, P = .435), and non-elective admission (8.5% vs 8.2%, P = .862) across patients with and without HF in the PSM cohort. Additionally, the prior disparate comorbidities in the crude analysis including coagulopathy, COPD, anemia, depression, electrolyte abnormalities, hypertension, hypothyroidism, liver disease, metastatic cancer, obesity, peptic ulcer, PVD, renal failure, and rheumatoid arthritis were similar after propensity matching, as demonstrated in Figure 3B.
Outcomes of the PSM cohort during index admission are shown in Figure 4 and Supplemental Figure 1B. Using PSM, the adjusted odds of mortality, major bleeding, and AKI during index hospitalization were still significantly higher in the HF group. There was no significant difference in the risk of periprocedural stroke, pericardial effusion, or cardiac tamponade. The mean length of stay of the index hospitalization was greater for the HF group compared with the no-HF group (1.66 ± 2.91 days vs 1.37 ± 1.94 days, P < .001). The mean cost of index hospitalization was also greater for the HF group compared with the no-HF group ($27 019 ± $13 504 vs $25 571 ± $11 930, P < .001). The mean C2V score for patients with HF was 4.52 ± 1.18, while the mean C2V score for patients without HF was 3.38 ± 1.12 (P < .001).
Outcomes with propensity matching at 30-day readmission
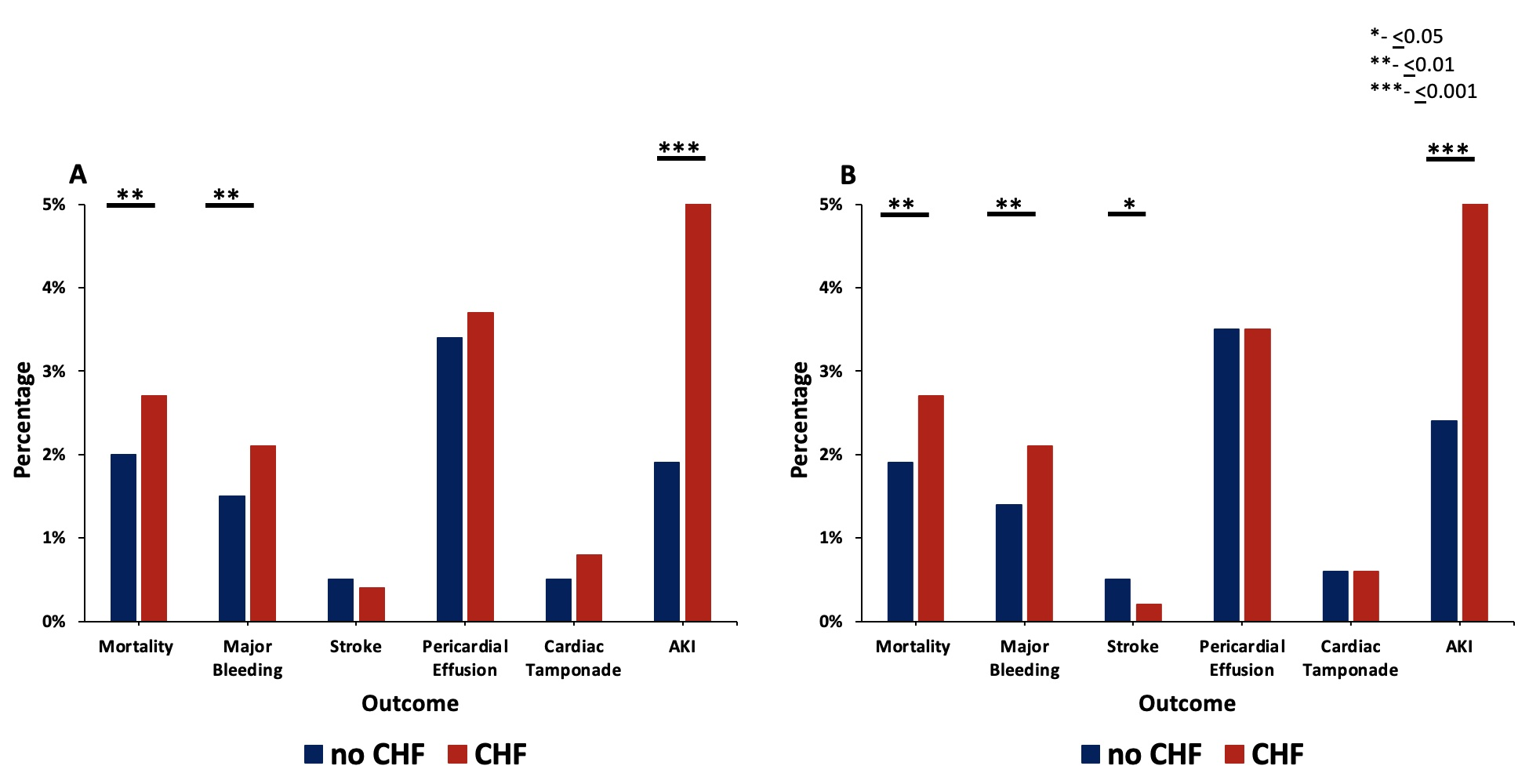
PSM analysis yielded a subcohort of 10 395 patients (5209 with HF and 5185 without HF) at 30-day readmission. As described above, prior disparate comorbidities were similar after PSM at 30-day readmission, as shown in the Supplemental Table. Similar to index admission, HF was associated with higher odds of mortality, major bleeding, and AKI (Figure 4, Supplemental Figure 2) Similarly, the odds of pericardial effusion and cardiac tamponade were not significantly different between the 2 PSM groups. The odds of stroke were significantly lower in the HF group at 30-day readmission in PSM analysis (HF: 11 patients with stroke vs no HF: 24 patients with stroke, OR 0.47 [0.23-0.96], P = .039). As expected, the mean C2V for patients with HF was higher at 4.59 ± 1.18 vs 3.47 ± 1.14 (P < .001). The monthly risk of stroke as estimated by the C2V score was compared with the actual stroke rate in our PSM sample and a 2.51-fold (60.2%) relative risk reduction in stroke risk was observed in patients with HF in the 30 days following LAAO.
Discussion
This retrospective nationwide study aims to evaluate the safety and efficacy of percutaneous LAAO in HF patients. LAAO closure has become more widespread due to evidence supporting the left atrial appendage (LAA) as the principal source of thrombus formation in AF-related stroke,11,12 significant reduction in stroke after surgical LAA closure,13,14 and non-inferiority of LAAO compared with OAC.8,9 However, there is a paucity of data comparing the outcomes of LAAO between AF with and without HF. A sub-study of the Left Atrial Thrombus on Transesophageal Echocardiography (LATTE) registry investigated thrombus in AF patients and found no difference in localization for left atrial (LA) thrombi for patients with HF (96.5% LAA vs 3.5% LA), despite the LA chamber being significantly larger. There was a progressively higher incidence of LA thrombi with worsening EF, and heart failure with reduced ejection fraction (HFrEF) was an important predictor of LA thrombus. Notably, this study found an increased risk of stroke in the HF group.15 Other studies demonstrate a relative risk of stroke of 1.4 to 3.1 in patients with AF and HF compared with AF alone.5,6 As such, LAAO would seem even more beneficial in HF patients compared with those without HF given the higher stroke risk in HF patients.
Our study demonstrated a significantly higher odds of mortality, major bleeding, and AKI in the HF cohort, despite propensity matching, without an increased risk of pericardial effusion, tamponade, and stroke following LAAO. These results are not surprising, given that HF conveys a significant risk to many of our measured outcomes. In a similar albeit smaller (n = 300) prospective study of percutaneous LAAO, HF did not increase the rate of procedural complications, and only mortality was higher in the HF cohort during long-term follow-up. Stroke and bleeding were not significantly higher; however, the event rate was notably low.16 Another similar large, exclusively inpatient study consistently found significantly worse outcomes in HF patients undergoing LAAO, with a mortality of 0.4% at discharge, an AKI rate of 5.3%, and a total stroke rate of 0.8%, consistent with our index admission data.17
Pericardial effusions are one of the most common complications following LAAO. Our observed incidence of 3.5% is lower than the initial Protect AF trial (with 4.8% requiring intervention), but higher than more recent studies showing pericardial effusion rates of 1.24% to 1.9%.18-26 The LAAO cases observed here were performed between 2015 and 2019 and likely reflect older generation devices and less operator experience compared with more recent studies, making the higher pericardial effusion rates unsurprising. Our study did not find an association of pericardial effusion/tamponade with HF status in LAAO patients within 30 days. As a feared complication of percutaneous LAAO, the lack of more pericardial effusions in HF patients again supports the safety of LAAO in this population.
On the other hand, HF was associated with higher odds of AKI following LAAO. AKI has long been known to result from increased filling pressures in HF patients via cardiorenal syndrome, which may be exacerbated by intraprocedural fluid and iodinated contrast administration. While AKI is not considered a common complication of LAAO, it becomes relevant when considering patients for same-day discharge. The AKI rate for all LAAO procedures at index admission in the present study was 2.3%. Many large retrospective studies show AKI rates from AF ablations to be markedly lower, at 0.23% to 0.40%, likely because of the lack of contrast.27-30 However, one study that stratified by patient frailty in AF ablation showed wide-ranging AKI rates from 4.4% in patients with low frailty to 42.5% in patients with higher frailty.31
Data on AKI rates for LAAO in the HF population specifically is sparse. At index admission, the AKI rate for the HF cohort was 3.9% with an OR of 2.11 (95% CI, 1.84-2.41; P < .001). Subsequently, we observed a 5.2% rate of AKI in our PSM-HF cohort at 30-day readmission with an OR of 2.24 (95% CI, 1.81-2.79; P < .001). While our data demonstrate higher prevalence of AKI following LAAO in HF patients, these rates are still less compared with other transcatheter procedures in the HF population (17.9% and 11.6% following transcatheter edge to edge repair and 15.4% and 9.9% following transcatheter aortic valve replacement have been reported for depressed and preserved LVEF, respectively).32-34 It is unclear in our analysis if this increased rate of AKI had further downstream consequences, such as dialysis requirements, which may be an area for further investigation. Altogether, our data suggest that clinicians should have a higher suspicion for AKI following LAAO in patients with HF.
Lastly, our study revealed no difference in stroke following index hospitalization. Surprisingly, a lower prevalence of stroke was observed in HF patients at 30-day readmission. This is in contrast to a large study of inpatients with HF undergoing LAAO wherein the ischemic stroke rate was significantly higher at discharge for HFrEF (0.2% vs 0.4%, P < .01), but HF with preserved ejection fraction (HFpEF) showed no difference from the non-HF sample (0.2% vs 0.2%).17 We suspect this finding may be due to type I statistical error resulting from a low event rate and borderline statistical significance (11 strokes composing 0.2% of the HF cohort vs 24 strokes composing 0.5% of the no HF cohort, P = .039); however, this may be explained by unaccounted-for cofounders, such as more patients in the HF cohort using OAC. While HF does not appear to predispose to a higher periprocedural stroke risk immediately following LAAO, long-term follow-up is undoubtedly necessary to shed light on the disparate reductions in stroke risk with LAAO among patients with and without HF.
Limitations
Our findings should be interpreted with certain limitations. This was a retrospective, observational study, and therefore we could only report temporal associations between LAAO and outcomes and cannot reliably describe causal relationships. Despite PSM using many clinical characteristics and comorbidities, HF surely carries an inherently higher risk of mortality and morbidity, and there may be covariates that were unaccounted for in our analysis. It is also important to note that the reported mortality rate in our study might be an underestimate because community events are not captured by NRD.
Also, our HF group was a combination of patients coded for acute and chronic, and systolic and diastolic HF. There are important distinctions between HFpEF, HFrEF, and other HF pathologies that may lead to different outcomes when it comes to LA thrombus and stroke risk, as seen by different rates of LA thrombus formation for HFpEF, HF with mid-range EF (HFmrEF), and HFrEF.15 Our study did not differentiate these populations and only stratified patients by whether or not they had a history of any type of HF. It is certainly possible that a subgroup analysis of HFrEF patients or any other group could provide different results.
Also, the NRD did not include data on medication use, so it is possible that certain cohorts may have unknowingly had a higher prevalence of OAC use during the study period. Given that NRD relies entirely on coding, the possibility of coding error cannot be excluded. Lastly, short follow-up captured by NRD prevented analysis of outcomes after greater than 30 days, which would be necessary to assess for stroke risk after LAAO.
Conclusions
Our study compares the safety profile of LAAO use in patients with HF to those without HF in the periprocedural period. Mortality, major bleeding, and AKI were elevated in the HF cohort given underlying morbidity and mortality from HF; however, HF was not associated with an increase in pericardial effusion or tamponade in this cohort. While peri-procedural stroke was not higher in patients with HF, further investigation with longer follow-up is required to evaluate whether the relative risk reduction of stroke following LAAO is similar between patients with and without HF.
Affiliations and Disclosures
Rafey Feroze, MD1; Alexander Cove, MD2; Yusef Saeed, MD2; Waqas Ullah, MD3; Nawaf Alhabdan, MD2; Marco Frazzetto, MD1; Nour Tashtish, MD1; Luis Augusto Palma Dallan, MD, PhD1; Steven J. Filby, MD1
From the 1Harrington Heart & Vascular Institute, University Hospitals, Cleveland, Ohio; 2Department of Medicine, University Hospitals, Cleveland, Ohio; 3Department of Cardiology, Thomas Jefferson Hospitals, Philadelphia, Pennsylvania.
Dr Feroze and Dr Cove contributed equally to the manuscript.
Disclosures: Dr Filby is a consultant for Boston Scientific. The remaining authors report no financial relationships or conflicts of interest regarding the content herein.
Address for correspondence: Steven J. Filby, MD, 11100 Euclid Avenue M.L. 5038, Cleveland, OH 44106, USA. Email: Steven.Filby@UHHospitals.org
Supplemental Material
References
1. Anter E, Jessup M, Callans DJ. Atrial fibrillation and heart failure: treatment considerations for a dual epidemic. Circulation. 2009;119(18):2516-2525. doi:10.1161/CIRCULATIONAHA.108.821306
2. Batul SA, Gopinathannair R. Atrial fibrillation in heart failure: a therapeutic challenge of our times. Korean Circ J. 2017;47(5):644-662. doi:10.4070/kcj.2017.0040
3. Diaz J, Martinez F, Calderon JM, et al. Incidence and impact of atrial fibrillation in heart failure patients: real-world data in a large community. ESC Heart Fail. 2022;9(6):4230-4239. doi:10.1002/ehf2.14124
4. Bergau L, Bengel P, Sciacca V, Fink T, Sohns C, Sommer P. Atrial fibrillation and heart failure. J Clin Med. 2022;11(9):2510. doi:10.3390/jcm11092510
5. Risk factors for stroke and efficacy of antithrombotic therapy in atrial fibrillation. Analysis of pooled data from five randomized controlled trials. Arch Intern Med. 1994;154(13):1449-1457. doi:10.1001/archinte.1994.00420130036007
6. Agarwal M, Apostolakis S, Lane DA, Lip GY. The impact of heart failure and left ventricular dysfunction in predicting stroke, thromboembolism, and mortality in atrial fibrillation patients: a systematic review. Clin Ther. 2014;36(9):1135-1144. doi:10.1016/j.clinthera.2014.07.015
7. Huded C, Krishnaswamy A, Kapadia S. Percutaneous left atrial appendage closure: is there a role in valvular atrial fibrillation. J Atr Fibrillation. 2017;9(5):1524. doi:10.4022/jafib.1524
8. Holmes DR Jr, Kar S, Price MJ, et al. Prospective randomized evaluation of the Watchman Left Atrial Appendage Closure device in patients with atrial fibrillation versus long-term warfarin therapy: the PREVAIL trial. J Am Coll Cardiol. 2014;64(1):1-12. doi:10.1016/j.jacc.2014.04.029
9. Reddy VY, Sievert H, Halperin J, et al; PROTECT AF Steering Committee and Investigators. Percutaneous left atrial appendage closure vs warfarin for atrial fibrillation: a randomized clinical trial. JAMA. 2014;312(19):1988-1998. doi:10.1001/jama.2014.15192
10. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613-619. doi:10.1016/0895-4356(92)90133-8
11. Manning WJ, Silverman DI, Waksmonski CA, Oettgen P, Douglas PS. Prevalence of residual left atrial thrombi among patients with acute thromboembolism and newly recognized atrial fibrillation. Arch Intern Med. 1995;155(20):2193-2198 doi:10.1001/archinte.1995.00430200078011
12. Shirani J, Alaeddini J. Structural remodeling of the left atrial appendage in patients with chronic non-valvular atrial fibrillation: implications for thrombus formation, systemic embolism, and assessment by transesophageal echocardiography. Cardiovasc Pathol. 2000;9(2):95-101. doi:10.1016/s1054-8807(00)00030-2
13. Whitlock RP, Belley-Cote EP, Paparella D, et al; LAAOS III Investigators. Left atrial appendage occlusion during cardiac surgery to prevent stroke. N Engl J Med. 2021;384(22):2081-2091. doi:10.1056/NEJMoa2101897
14. Tsai YC, Phan K, Munkholm-Larsen S, Tian DH, La Meir M, Yan TD. Surgical left atrial appendage occlusion during cardiac surgery for patients with atrial fibrillation: a meta-analysis. Eur J Cardiothorac Surg. 2015;47(5):847-854. doi:10.1093/ejcts/ezu291
15. Wybraniec MT, Mizia-Szubryt M, Cichoń M, et al. Heart failure and the risk of left atrial thrombus formation in patients with atrial fibrillation or atrial flutter. ESC Heart Fail. 2022;9(6):4064-4076. doi:10.1002/ehf2.14105
16. Saad M, Osman M, Hasan‐Ali H, et al. Atrial appendage closure in patients with heart failure and atrial fibrillation: industry-independent single-centre study. ESC Heart Fail. 2022;9(1):648-655. doi:10.1002/ehf2.13698
17. Munir MB, Khan MZ, Darden D, et al. Association of heart failure with procedural complications and in-hospital outcomes from left atrial appendage occlusion device implantation in patients with atrial fibrillation: insights from the national inpatient sample of 62 980 procedures. Europace. 2022;24(9):1451-1459. doi:10.1093/europace/euac043
18. Ledesma PA, Uzomah UA, Yu X, et al. MAUDE database analysis of post-approval outcomes following left atrial appendage closure with the Watchman device. Am J Cardiol. 2021;152:78-87. doi:10.1016/j.amjcard.2021.04.0155
19. Price MJ, Valderrábano M, Zimmerman S, et al. Periprocedural pericardial effusion complicating transcatheter left atrial appendage occlusion: a report from the NCDR LAAO registry. Circ Cardiovasc Interv. 2022;15(5):e011718. doi:10.1161/CIRCINTERVENTIONS.121.011718
20. Munir MB, Khan MZ, Darden D, et al. Pericardial effusion requiring intervention in patients undergoing percutaneous left atrial appendage occlusion: prevalence, predictors, and associated in-hospital adverse events from 17,700 procedures in the United States. Heart Rhythm. 2021;18(9):1508-1515. doi:10.1016/j.hrthm.2021.05.017
21. Schmidt B, Betts TR, Sievert H, et al. Incidence of pericardial effusion after left atrial appendage closure: the impact of underlying heart rhythm-data from the EWOLUTION study. J Cardiovasc Electrophysiol. 2018;29(7):973-978. doi:10.1111/jce.13626
22. Mogalapalli A, Kumar S, Lobo T, et al. Delayed pericardial effusion following left atrial appendage closure: a 5-year single-center experience. J Invasive Cardiol. 2023;35(1):E1-E6. doi:10.25270/jic/22.00181
23. Holmes DR Jr, Korsholm K, Rodés-Cabau J, Saw J, Berti S, Alkhouli MA. Left atrial appendage occlusion. EuroIntervention. 2023;18(13):e1038-e1065. doi:10.4244/EIJ-D-22-00627
24. Khalil F, Arora S, Killu AM, et al. Utilization and procedural adverse outcomes associated with Watchman device implantation. Europace. 2021;23(2):247-253. doi:10.1093/europace/euaa219
25. Kar S, Doshi SK, Sadhu A, et al; PINNACLE FLX Investigators. Primary outcome evaluation of a next-generation left atrial appendage closure device: results from the PINNACLE FLX trial. Circulation. 2021;143(18):1754-1762. doi:10.1161/CIRCULATIONAHA.120.050117
26. Reddy VY, Gibson DN, Kar S, et al. Post-approval U.S. Experience with left atrial appendage closure for stroke prevention in atrial fibrillation. J Am Coll Cardiol. 2017;69(3):253-261. doi:10.1016/j.jacc.2016.10.010
27. Ngo L, Ali A, Ganesan A, Woodman R, Adams R, Ranasinghe I. Gender differences in complications following catheter ablation of atrial fibrillation. Eur Heart J Qual Care Clin Outcomes. 2021;7(5):458-467. doi:10.1093/ehjqcco/qcab035
28. Wu L, Narasimhan B, Ho KS, Zheng Y, Shah AN, Kantharia BK. Safety and complications of catheter ablation for atrial fibrillation: predictors of complications from an updated analysis the National Inpatient database. J Cardiovasc Electrophysiol. 2021;32(4):1024-1034. doi:10.1111/jce.14979
29. Ngo L, Ali A, Ganesan A, et al. Institutional variation in 30-day complications following catheter ablation of atrial fibrillation. J Am Heart Assoc. 2022;11(4):e022009. doi:10.1161/JAHA.121.022009
30. Ngo L, Ali A, Ganesan A, Woodman R, Adams R, Ranasinghe I. Ten-year trends in mortality and complications following catheter ablation of atrial fibrillation. Eur Heart J Qual Care Clin Outcomes. 2022;8(4):398-408. doi:10.1093/ehjqcco/qcab102
31. Ali S, Kumar M, Khlidj Y, et al. Impact of frailty in hospitalized patients undergoing catheter ablation for atrial fibrillation. J Cardiovasc Electrophysiol. 2024;35(10):1929-1938. doi:10.1111/jce.16383
32. Krittanawong C, Hahn J, Virk HUH, et al. In-hospital complications after MitraClip in patients with heart failure and preserved versus reduced ejection fraction in the United States. Cardiovasc Revasc Med. 2024;62:34-39. doi:10.1016/j.carrev.2023.11.017
33. Abbas S, Qayum I, Wahid R, et al. Acute kidney injury in transcatheter aortic valve replacement. Cureus. 2021;13(5):e15154. doi:10.7759/cureus.15154
34. Fatuyi M, Akinti S, Rukayat O, et al. Systolic heart failure is associated with higher mortality among patients undergoing transcatheter aortic valve replacement: a nationwide analysis. Curr Probl Cardiol. 2023;48(12):101936. doi:10.1016/j.cpcardiol.2023.101936





















