Ocular Radiation Exposure of the Primary Operator Performing Selective Coronary Angiography From a Radial Artery Approach
© 2025 HMP Global. All Rights Reserved.
Any views and opinions expressed are those of the author(s) and/or participants and do not necessarily reflect the views, policy, or position of the Journal of Invasive Cardiology or HMP Global, their employees, and affiliates.
Abstract
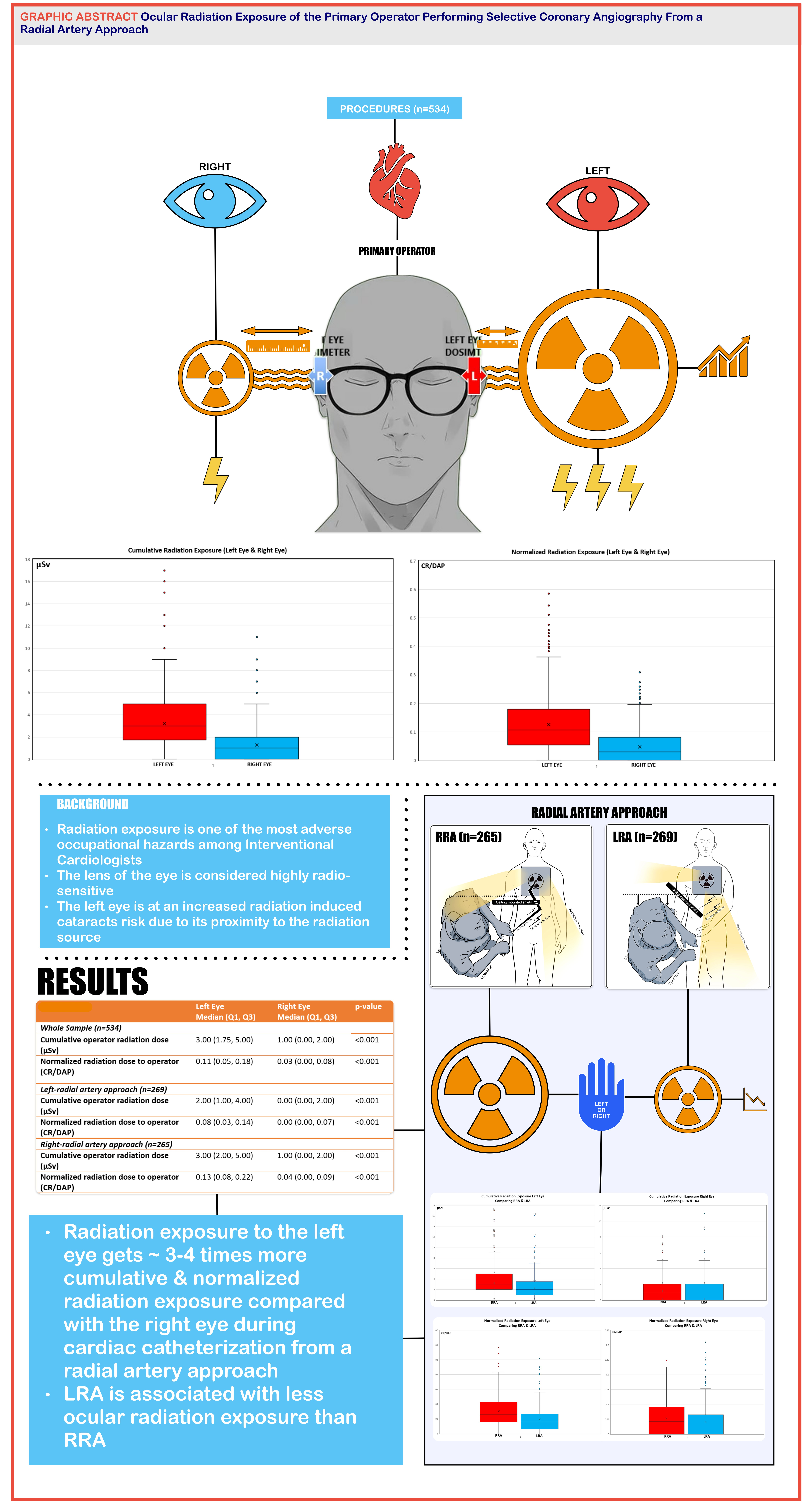
Background. Radiation exposure is one of the most adverse occupational hazards faced by interventional cardiologists. The lens of the eye is considered highly radiosensitive. The left eye (LE) is at considerably more risk of radiation-induced cataracts than the right eye (RE) due to its proximity to the radiation source during cardiac catheterization.
Methods. This single-center retrospective study (n = 534) assessed the cumulative radiation (CR) exposure in microsieverts (μSv) and the normalized radiation exposure (CR/DAP) to the LE and RE of the primary operator when using a radial artery approach.
Results. In the whole sample, median CR (LE: 3 vs RE: 1, P < .001) and median CR/DAP (LE: 0.11 vs RE: 0.03, P < .001) were higher for the LE than the RE. This same pattern occurred in separate analyses of both radial artery approaches, regardless of patient sex, height, or body mass index.
Conclusions. Radiation exposure to the LE compared with the RE of interventional cardiologists is significantly more during selective coronary angiography using a radial artery approach. The authors recommend the use of radioprotective glasses in conjunction with an optimally placed ceiling-mounted shield to minimize ocular radiation exposure.
Introduction
Radiation exposure is one of the most adverse occupational hazards faced by interventional cardiologists. The lens of the eye is considered highly radiosensitive because its cells are specialized and have limited repair mechanisms, making it particularly susceptible to damage from ionizing radiation.1 There is an increased incidence of cataracts in interventional cardiologists.2 The left eye (LE) is at significantly more risk for radiation-induced cataracts, as studies have shown a higher prevalence in the LE compared with the right eye (RE).3
The radial artery approach is increasingly implemented in the United States. Cardiac catheterization from the radial approach decreases bleeding complications for patients as well as reduces institutional costs.4,5 Unfortunately, greater radiation exposure is concomitant with the use of a radial artery approach compared with a femoral artery approach, even when performed by expert operators.6 This can place more radiosensitive organs, such as the eyes, at greater risk for radiation-induced illnesses.
Operators generally operate on the right side of the patient because of historical laboratory configurations, which place the LE in closer proximity to the radiation source. One study evaluated the difference in the cumulative amount of radiation exposure by comparing the LE to the RE during cardiac catheterization and found higher exposure to the LE.7 However, to the best of our knowledge, no study has evaluated ocular radiation exposure specifically from a radial artery approach. This retrospective study evaluates the amount of ocular radiation exposure from a radial artery approach during cardiac catheterization.
Methods
Setting
This is a retrospective secondary analysis from a randomized clinical trial8 conducted at a tertiary center from November 2022 through February 2024. We included patients 18 years or older who were undergoing elective cardiac catheterization suitable for a radial approach. Patients who had hemodynamic instability, non-palpable radial pulses, ST-segment or non-ST-segment elevation myocardial infarction, previous coronary artery bypass grafts, and arteriovenous fistulas were excluded. The study received ethical approval from the Maimonides Medical Center institutional review board, and informed consent was obtained from both patients and operators.
Radiation to the operator was measured for the diagnostic portion of the catheterization. Dosimeters were removed if the procedure proceeded to a percutaneous coronary intervention (PCI), physiological assessment, or intravascular ultrasound. A total of 13 operators with 4 to 30 years of experience participated.
Procedures
All procedures were performed on the right side of the patient. The radial artery was cannulated using a modified Seldinger technique. Two standard right coronary artery projections and 4 standard left coronary projections were taken. Additional projections/cineangiograms were taken at the operator’s discretion. The lowest degree of angulation necessary to obtain diagnostic coronary angiograms was used with the aim of reducing operator and patient radiation exposure. Catheters were selected according to the operator’s preference.
Radial setup
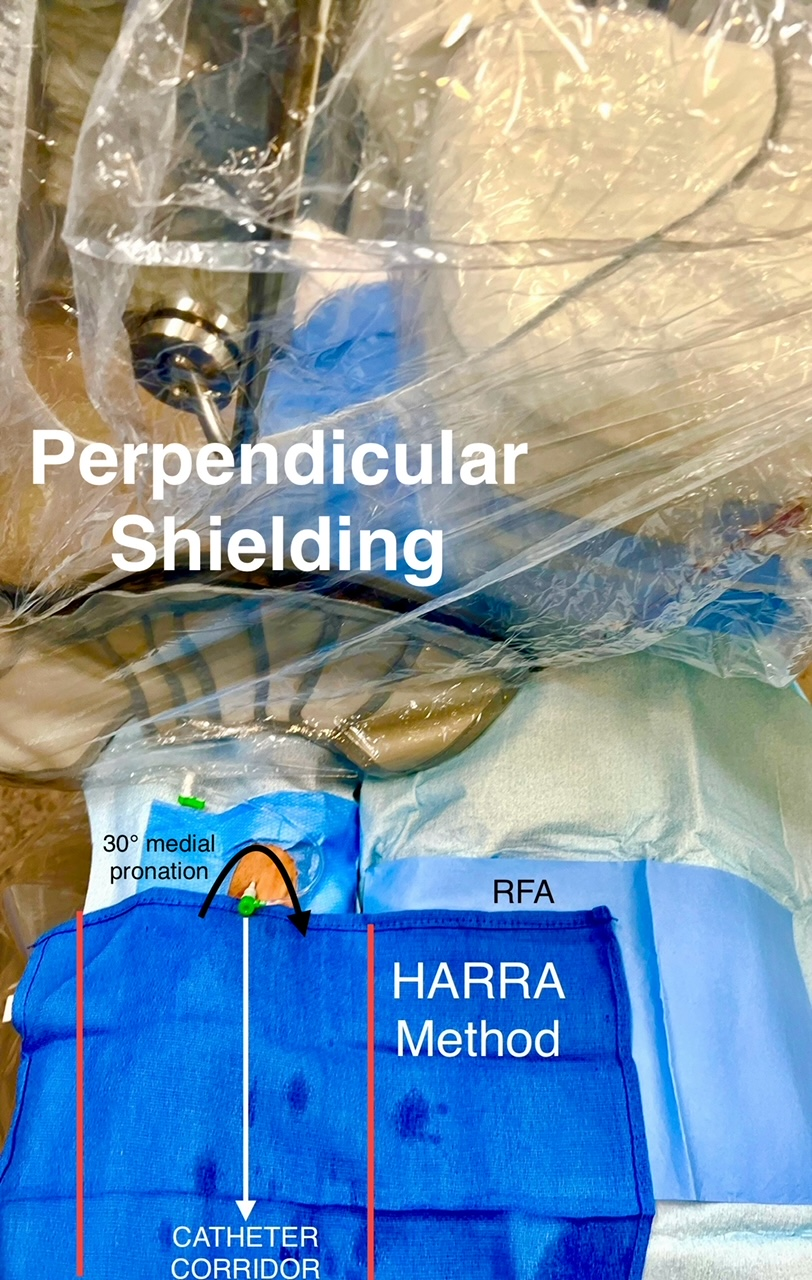
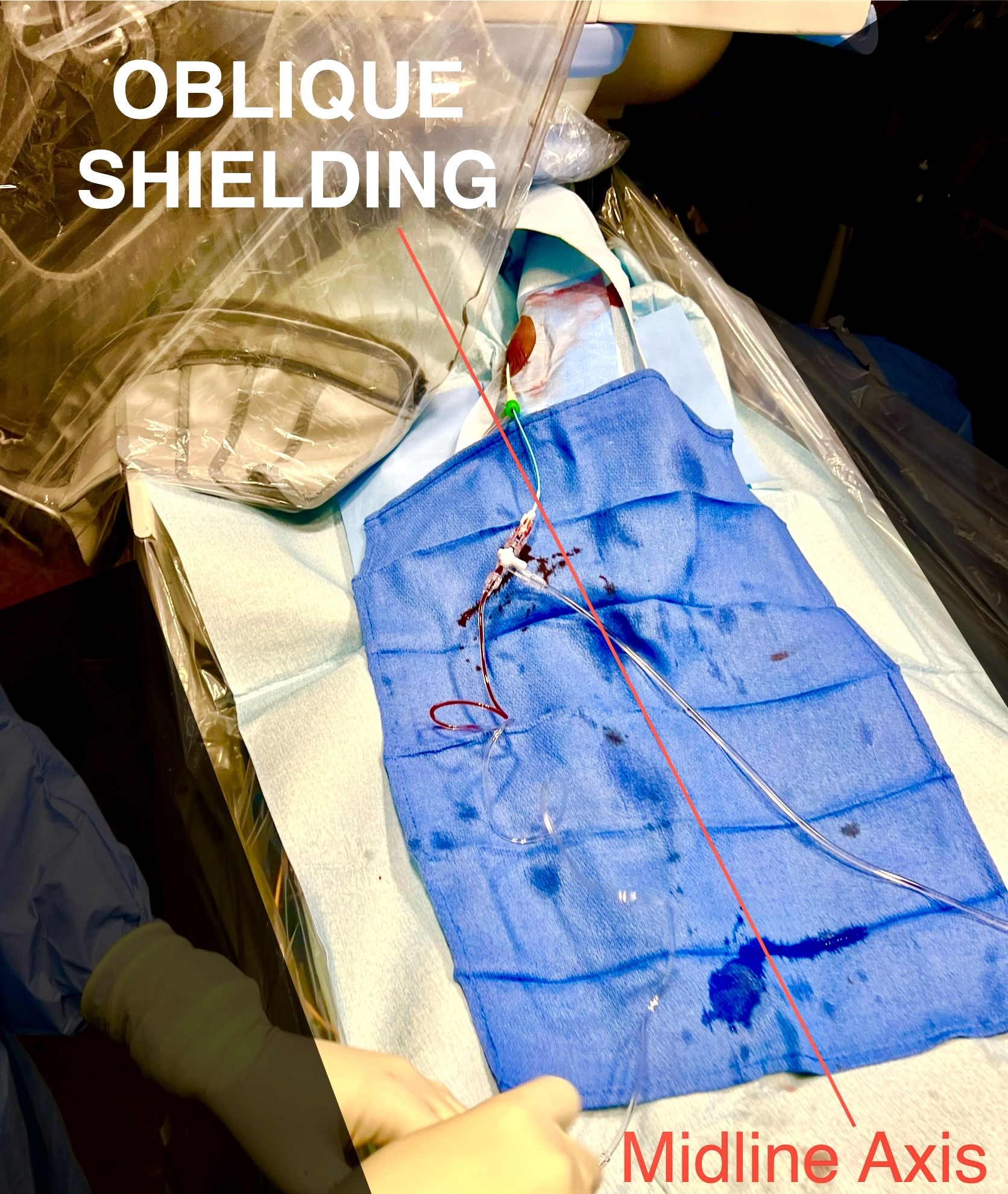
Both right-radial artery (RRA) and left-radial artery (LRA) approaches were utilized during this study. For the RRA, a hyper-adduction setup was used for each participant. The RRA was positioned as close as possible to the right flank of the body, as this approach has shown to yield lower operator radiation than a conventional RRA setup (Figure 1).9 For the LRA, the patients’ arm was adducted to place the hub of the sheath as close as possible to the patient’s midline axis after access. The left arm was draped with a Radial Access Sleeve (Tesslagra Design Solutions) and secured toward the operator with clamps to maintain ergonomics (Figure 2). These setups have been previously described.8
Radiation measurements and radiation safety measures
Two real-time radiation dosimeters (RaySafe i3; RaySafe) were placed outside the operator’s radiation protective glasses (RPG) at the LE and RE. Radiation was measured in microsieverts (µSv). The radiation-dose measurements were transmitted to a portable bedside monitor. Operators could not see the real-time dosimetry monitor. All operators wore identical radiation protection garments. A ceiling-mounted lead shield outfitted with a lead skirt that contoured to the patient’s body was used to block scatter radiation. Two fluoroscopic suites (GE Innova 2100 and 3100) were used. Both had identical shielding arrangements and software generating the same radiation output. No adjunctive technologies were utilized. Collimation was employed habitually, as is typical practice within our cardiac catheterization laboratory.
Study outcomes
The primary outcomes were comparisons between the LE and RE for (1) the cumulative radiation exposure (CR) of the primary operator, expressed in microsieverts (μSv); and (2) the cumulative operator radiation dose normalized by dose area product (DAP), expressed as CR/DAP.
The secondary outcomes were these analyses repeated in separate strata of the radial artery approach (left or right) and patient sex, height (≤ 175.4 cm or > 175.4 cm; the cutoff value was based on the 50th percentile of male height in the United States of 175.4 cm),10 and body mass index (BMI) (< 25.0 kg/m2, 25.0-29.9 kg/m2, or > 30.0 kg/m2), using the standard adult categories for normal weight, overweight, and obese.11
We described patient demographics (age, sex, and race/ethnicity), measurements (height, weight, BMI, and body surface area), and history (smoking, hypertension, diabetes, and chronic kidney disease). We also described procedural characteristics of DAP, fluoroscopy time, contrast amount, milligray, number of cineangiograms, and number of catheters used.
Statistical analysis
The mean and SD or median and first and third quartile described the continuous variables. The frequency and percentage described the categorical variables. As the data had a skewed distribution, the Wilcoxon signed-rank test compared the continuous variables. All P-values were 2-tailed with alpha level for significance at a P-value of less than 0.05. IBM SPSS Statistics Version 29.0 was used for the analyses (IBM Corporation).
Results
Table 1 shows the sample characteristics (n = 534). The mean age of the patients was slightly over 67 years, almost one-third were male, and more than half were of non-white race/ethnicity. The mean height was almost 169 cm, and the mean BMI was almost 30 kg/m2. Slightly more than one-fifth smoked. Almost 90% had hypertension. Procedure variables included a mean DAP of 27.20 cGy·cm2, fluoroscopy time of 3.5 minutes, contrast amount delivered of 47.9 (cc), and milligray of 547.5.
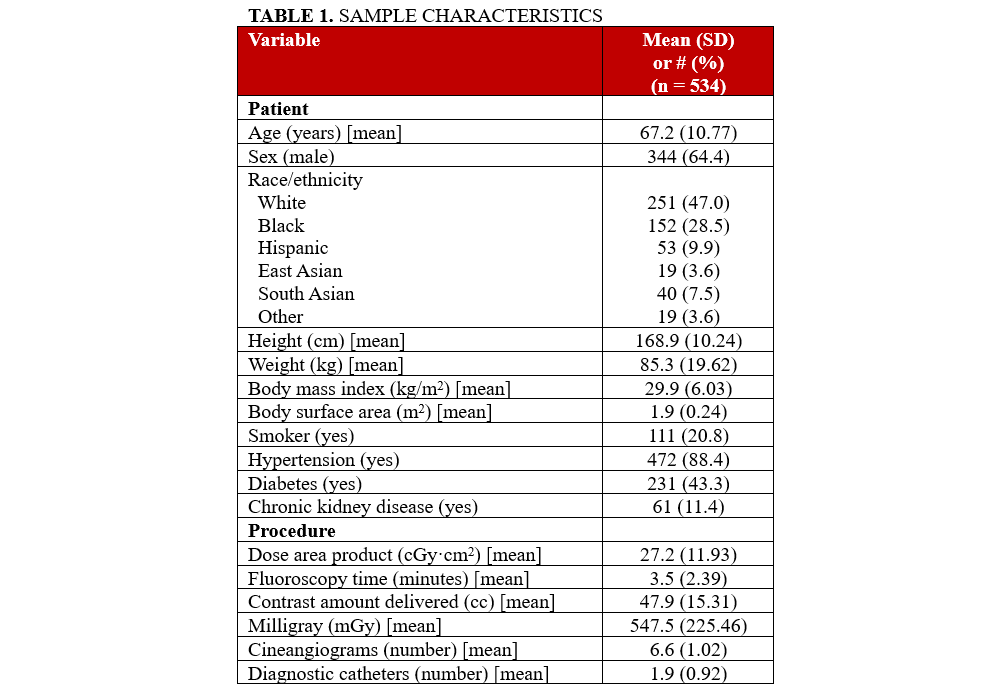
Table 2 shows comparisons between the LE and RE for cumulative CR and CR/DAP in the whole sample and for radial artery approach (Figures 3 and 4). In the whole sample, median CR (P < .001) and CR/DAP (P < .001) were significantly higher for the LE than the RE. For both the LRA and the RRA, the median CR (all P < .001) and median CR/DAP (all P < .001) were each significantly higher for the LE than the RE. Access-site crossover where the initial radial artery site was switched after starting the procedure occurred for 1 patient originally randomized to the LRA and for 3 patients originally randomized to the RRA.
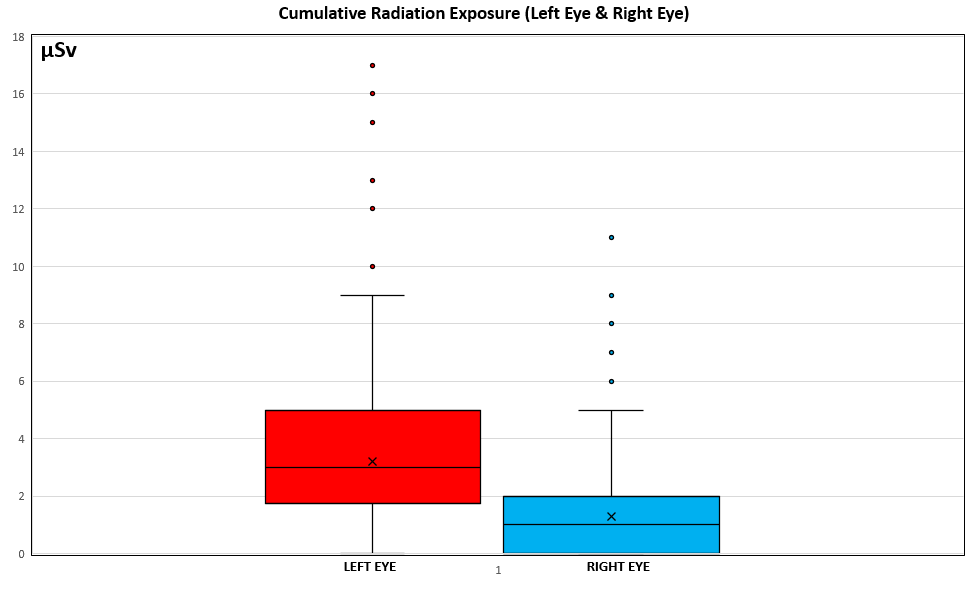
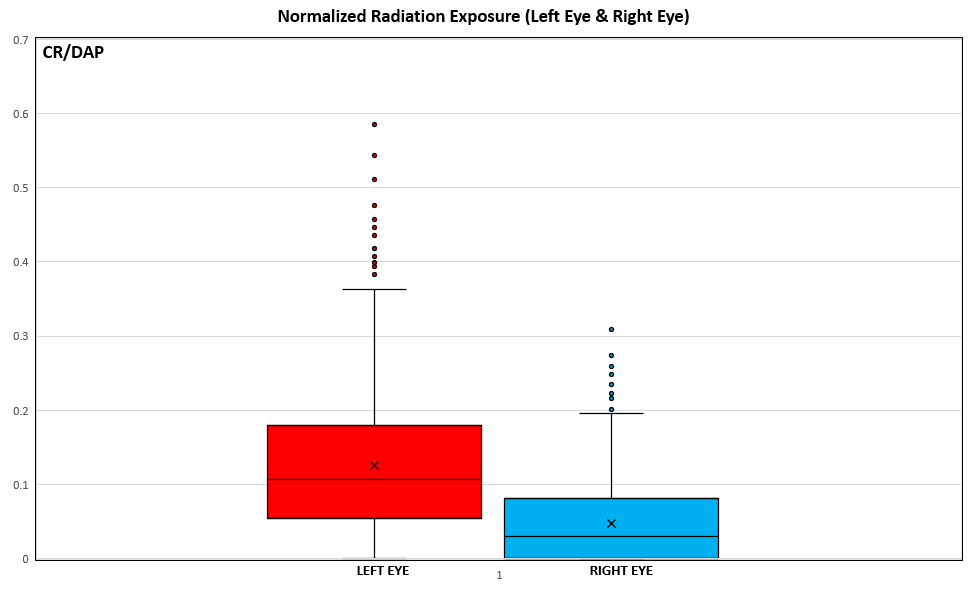
Table 3 shows comparisons between the LE and RE for CR and CR/DAP in terms of the patient characteristics. For both females and males, the median CR (all P < .001) and median CR/DAP (all P < .001) were each significantly higher for the LE than the RE. In terms of patient height, for patients 175.4 cm or shorter and patients taller than 175.4 cm, the median CR (all P < .001) and median CR/DAP (all P < .001) were each significantly higher for the LE than the RE. In terms of BMI, for patients with a BMI of less than 25.0 kg/m2, between 25.0 and 29.9 kg/m2, and greater than 30.0 kg/m2, the median CR (all P < .001) and median CR/DAP (all P < .001) were each significantly higher for the LE than the RE. The 4 underweight patients were included in the normal weight category.
The Kruskal-Wallis test compared BMI categories. CR significantly differed (P < .001) for the LE for patients who had a BMI of less than 25 kg/m2 had lower median CR than those with a BMI between 25.0 and 29.9 kg/m2 and a BMI of greater than 30 kg/m2. In terms of the RE, the CR significantly differed (P < .001) for patients with a BMI of less than 25 kg/m2, and patients with a BMI between 25.0 and 29.9 kg/m2 had a lower median CR than those with a BMI of greater than 30 kg/m2. However, the operators’ CR/DAP did not significantly differ between BMI categories for the LE (P = .40) and RE (P = .08).
Discussion
This study provides an evaluation of the level of ocular radiation exposure experienced by seasoned interventional cardiologists in the cardiac catheterization laboratory. Furthermore, it assesses ocular exposure specifically from a radial artery approach, which has become the default access site for most operators. This study found greater radiation exposure for the LE compared with the RE. These exposure patterns were the same regardless of patient sex, height, or BMI. These findings are understandable in terms of the operators’ position in relation to the patient and fluoroscopic unit; the LE is in direct line with, and in closer proximity to, the scatter radiation source and the x-ray tube, putting it at a higher radiation-induced risk of cataracts. Our study also found that patients with a BMI of greater than 30 kg/m2 had the greatest cumulative radiation exposure in both the LE and RE. This reinforces prior literature regarding increased exposure with larger patients and stresses the need for vigilant radiation protection for the operators working with those patients.12
Similar to previous research, our study shows more exposure to the LE compared with the RE.7 However, we found less overall exposure to the eyes, presumably because our study evaluated diagnostic coronary angiography, not PCIs. Furthermore, the arterial access site was not specified in their study, which can affect exposure. There are several other factors that can affect radiation exposure to the eyes that should also be taken into account; elements such as fluoroscopic settings, number of cineangiograms, angiographic projection angles, operator position and experience, and use of adjunctive or dedicated radiation protection technologies can all contribute to radiation dose.
Exposure patterns may differ between left and right radial approaches. Our clinical trial8 showed less exposure to each eye from an LRA approach. This is presumably due to better shielding with the LRA compared with the RRA. Our institution facilitates an oblique shield position, whereas, for the RRA setup, the shield is typically in a more perpendicular position. Operators using the LRA may have positioned the patient table lower than operators using the RRA, which creates a geometrical advantage between the radiation scatter source and eye dosimeters. The LRA may also allow for more advantageous aorto-subclavian entry approaches than the RRA, as the aorto-innominate junction entry angle may be more technically challenging for selective coronary angiography.
Protective measures
Radiation protective glasses (RPG) can be effective at reducing radiation exposure to the eyes of operators, but there are some limitations. RPG can reduce eye radiation by 35% to 90%.13 However, some studies have found that standard RPG are increasingly ineffective at reducing radiation exposure.14 For the LE, standard models of RPG only decrease the radiation reaching the most radiosensitive region of the eye lens by 22% or less.15 A study showed that, despite increasing the lead thickness from 0.4 to 0.75 mm, there was only a slight effect on the protection provided in the simulation of clinical use; adding lead to the frames did not add meaningful protection either.16 However, the use of ceiling-mounted shields with standard RPG significantly reduces ocular radiation exposure.17
Research has also examined differences in ocular radiation exposure with a half- or full-leaded visor or RPG. The full visor was shown to yield the most significant dose reductions.18 However, the caveat is that the visor is significantly heavier than a half visor or lead glasses. During long procedures, orthopedic strain on the neck can be a concern. This “trade-off” for increased protection should be considered carefully, as orthopedic injuries can be damaging to operators’ health. Other dedicated devices such as the Rampart (RAMPART ic, LLC) and Protego (Image Diagnostics, Inc.) radiation protection systems have also shown promising data regarding radiation reductions to the head while preserving orthopedic integrity.19,20
Surveys
The use of radioprotective eyewear and other shielding is not ubiquitous among operators; they are recommended but not mandated by professional societies or institutions. The Jacob study reports that approximately 50% of interventionalists use RPG.2 Another recent study found that roughly 30% of interventional cardiologists used RPG, and only roughly two-thirds used under-table lead shields and ceiling-mounted shields. An alarming 10.2% of respondents reported cataracts and 8.2% had malignancies. Furthermore, 25% did not wear dosimeters to monitor monthly radiation exposure.21
Recommendations
Our findings demonstrate the need for use of specific personal protective equipment for the eyes, particularly for the LE because of its proximity to the source of radiation. Additionally, there is a need to educate operators about the occupational risks associated with ionizing radiation exposure. Operators should be exceedingly vigilant with personal radiation protective measures for the eyes, particularly for patients with a BMI of greater than or equal to 30 kg/m2. We recommend the use of dedicated RPG in conjunction with optimally placed ceiling-mounted shields to minimize the amount of exposure to the eyes.
Strengths and limitations
A strength of this study is that, in a large sample size, we found significantly more radiation exposure to the LE compared with the RE from the radial artery approach, which has become the default approach for most operators. Another strength is that we demonstrate the normalized amount of radiation exposure, which accounts for procedural variations. We also report ocular radiation exposure from LRA and RRA. This study also has several limitations. First, the study was conducted at a single center and, therefore, the results may not be generalizable to other centers. Second, ceiling-mounted shield placement may differ between operators, which may affect the radiation exposure of individual operators. Third, we did not account for the operators’ height, which can yield different exposure patterns. Future research may investigate these differences to determine whether they are associated with different radiation exposure patterns.
Conclusions
During selective coronary angiography performed via the radial artery approach, interventional cardiologists experience significantly greater radiation exposure to the LE compared with the RE. We recommend the use of radioprotective glasses in conjunction with an optimally placed ceiling-mounted shield to minimize ocular radiation exposure.
Affiliations and Disclosures
Richard Casazza, MAS1; Bilal Malik, MD1; Arsalan Hashmi, MD1; Joshua Fogel, PhD2; Enrico Montagna, RT (CI)1; Darren Gibson, RT1; Andres Palacio, RT (CI)1; Habiba Beginyazova, RT1; Robert Frankel, MD1; Jacob Shani, MD1
From the 1Department of Cardiology, Maimonides Medical Center, Brooklyn, New York; 2Department of Management, Marketing, and Entrepreneurship, Brooklyn College, Brooklyn, New York.
Disclosures: Mr Casazza is the director of research and development at Tesslagra Design Solutions. The remaining authors report no financial relationships or conflicts of interest regarding the content herein.
Address for correspondence: Richard Casazza, MAS, Department of Cardiology, Maimonides Medical Center, 4802 10th Avenue, Brooklyn, NY 11219, USA. Email: rcasazza@maimonidesmed.org; X: @tesslagra
References
- Hamada N. Ionizing radiation sensitivity of the ocular lens and its dose rate dependence. Int J Radiat Biol. 2017;93(10):1024-1034. doi:10.1080/09553002.2016.1266407
- Jacob S, Boveda S, Bar O, et al. Interventional cardiologists and risk of radiation-induced cataract: results of a French multicenter observational study. Int J Cardiol. 2013;167(5):1843-1847. doi:10.1016/j.ijcard.2012.04.124
- Rose A, Rae WID, Sweetlove MA, Ngetu L, Benadjaoud MA, Marais W. Radiation induced cataracts in interventionalists occupationally exposed to ionising radiation. SA J Radiol. 2022;26(1):2495. doi:10.4102/sajr.v26i1.2495
- Rinfret S, Kennedy WA, Lachaine J, et al. Economic impact of same-day discharge after uncomplicated transradial percutaneous coronary intervention and bolus-only abciximab regimen. JACC Cardiovasc Interv. 2010;3(10):1011-1019. doi:10.1016/j.jcin.2010.07.011
- Sciahbasi A, Frigoli E, Sarandrea A, et al. Radiation exposure and vascular access in acute coronary syndromes: the RAD-Matrix trial. J Am Coll Cardiol. 2017;69(20):2530-2537. doi:10.1016/j.jacc.2017.03.018
- Sciahbasi A, Rigattieri S, Sarandrea A, et al. Determinants of operator radiation exposure during percutaneous coronary procedures. Am Heart J. 2017;187:10-18. doi:10.1016/j.ahj.2017.02.012
- Alnaaimi M, Alduaij M, Shenawy F, et al. Assessment of eye doses to staff involved in interventional cardiology procedures in Kuwait. Radiat Environ Biophys. 2021;60(4):639-645. doi:10.1007/s00411-021-00929-3
- Casazza R, Malik B, Hashmi A, et al. Operator radiation exposure comparing the left radial artery approach and a uniform hyper-adducted right radial artery approach: the HARRA study. Circ Cardiovasc Interv. 2025;18(4):e014602. doi:10.1161/CIRCINTERVENTIONS.124.014602
- Sciahbasi A, Frigoli E, Sarandrea A, et al. Determinants of radiation dose during right transradial access: insights from the RAD-MATRIX study. Am Heart J. 2018; 196:113-118. doi:10.1016/j.ahj.2017.10.014
- Fryar CD, Carroll MD, Gu Q, Afful J, Ogden CL. Anthropometric reference data for children and adults: United States, 2015-2018. National Center for Health Statistics, U.S. Department of Health and Human Services; 2021. Accessed December 22, 2024. https://www.cdc.gov/nchs/data/series/sr_03/sr03-046-508.pdf
- U.S. Centers for Disease Control and Prevention. Adult BMI categories. Accessed December 22, 2024. https://www.cdc.gov/bmi/adult-calculator/bmi-categories.html
- Madder RD, VanOosterhout S, Mulder A, et al. Patient body mass index and physician radiation dose during coronary angiography. Circ Cardiovasc Interv. 2019;12(1):e006823. doi:10.1161/CIRCINTERVENTIONS.118.006823
- Gutierrez-Barrios A, Cañadas-Pruaño D, Noval-Morillas I, Gheorghe L, Zayas-Rueda R, Calle-Perez G. Radiation protection for the interventional cardiologist: practical approach and innovations. World J Cardiol. 2022;14(1):1-12. doi:10.4330/wjc.v14.i1.1
- Ciraj-Bjelac O, Rehani MM, Sim KH, Liew HB, Vano E, Kleiman NJ. Risk for radiation-induced cataract for staff in interventional cardiology: is there reason for concern? Catheter Cardiovasc Interv. 2010;76(6):826-834. doi:10.1002/ccd.22670
- Honorio da Silva E, Martin CJ, Vanhavere F, Dabin J, Buls N. An investigation into potential improvements in the design of lead glasses for protecting the eyes of interventional cardiologists. J Radiol Prot. 2022;42(3). doi:10.1088/1361-6498/ac758f
- Kirkwood ML, Klein A, Guild J, et al. Novel modification to leaded eyewear results in significant operator eye radiation dose reduction. J Vasc Surg. 2020;72(6):2139-2144. doi:10.1016/j.jvs.2020.02.049.
- Martin CJ. Eye lens dosimetry for fluoroscopically guided clinical procedures: practical approaches to protection and dose monitoring. Radiat Prot Dosimetry. 2016;169(1-4):286-291. doi:10.1093/rpd/ncv431
- Samara ET, Cester D, Furlan M, Pfammatter T, Frauenfelder T, Stüssi A. Efficiency evaluation of leaded glasses and visors for eye lens dose reduction during fluoroscopy guided interventional procedures. Phys Med. 2022;100:129-134. doi:10.1016/j.ejmp.2022.06.021
- Lisko JC, Shekiladze N, Chamoun J, et al. Radiation exposure using rampart vs standard lead aprons and shields during invasive cardiovascular procedures. J Soc Cardiovasc Angiogr Interv. 2023;3(1):101184. doi:10.1016/j.jscai.2023.101184
- Dixon SR, Rabah M, Emerson S, Schultz C, Madder RD. A novel catheterization laboratory radiation shielding system: results of pre-clinical testing. Cardiovasc Revasc Med. 2022;36:51-55. doi:10.1016/j.carrev.2021.05.017
- Cader, F, Thamman, R, Burgess, S, et al. TCT-742 radiation hazards and safety among physicians and allied health practitioners (AHP) in invasive cardiology procedures. JACC. 2024;84 (18_Supplement) B295-B296. doi:10.1016/j.jacc.2024.09.887





















