Intravascular Brachytherapy for the Management of Drug-Eluting In-Stent Restenosis
© 2025 HMP Global. All Rights Reserved.
Any views and opinions expressed are those of the author(s) and/or participants and do not necessarily reflect the views, policy, or position of the Journal of Invasive Cardiology or HMP Global, their employees, and affiliates.
Abstract
Objectives. This study aimed to evaluate the utilization and clinical outcomes of coronary intravascular brachytherapy (IVBT) as a treatment modality for multilayer in-stent restenosis (ISR).
Methods. This multicenter study retrospectively analyzed 101 patients who presented for percutaneous intervention of recurrent drug-eluting stent ISR using IVBT from 2019 to 2023. The primary outcome assessed was target lesion revascularization (TLR) at 1 year. Secondary endpoints were aimed to establish procedural safety. All lesions had evaluation by either angiography, intravascular ultrasound, or both.
Results. The majority of ISR was related to neointimal hyperplasia (61.4%), with stent underexpansion (11.9%) being the second most common cause. The average layer of stents in the sample was 1.90 layers. Prior to delivery of IVBT, lesions were prepared with balloon angioplasty, laser atherectomy, intravascular lithotripsy, or a combination of pretreatment strategies. The average time of the IVBT dwell period was 11 minutes, with an average dose of 21.76 Gy. Of the 101 patients evaluated, TLR occurred in 10.9% of patients at 1 year. Readmission at 30 days was 4.9% and vascular complications occurred in 3.9% of the patients. Major adverse cardiac events were limited to 0.9% of the patients, and no peri-procedural myocardial infarctions, urgent need for revascularization within 24 hours, need for mechanical support, nor cardiac arrest were observed.
Conclusions. In this study, IVBT proved to be a safe and effective treatment modality for multilayer ISR. The study generates the hypothesis for the routine use of IVBT in this commonly encountered clinical scenario. Larger and prospective randomized studies are needed.
Introduction
Drug-eluting stents (DES) are the current standard of care for managing obstructive coronary artery disease requiring percutaneous intervention. However, mechanical, biological, and patient-specific factors can lead to neointimal hyperplasia or neoatherosclerosis, which might result in in-stent restenosis (ISR).1 ISR can cause a recurrence of ischemic symptoms, frequently necessitating repeated intervention with DES. It also accounts for approximately 10% of percutaneous coronary interventions (PCI) in the United States.2 Recurrent ISR presents greater challenges, with repeat failure rates of 12% to 14% in low-risk lesions, and up to 41% at 1 year for cases involving 3 or more stent layers.1-3
Intravascular brachytherapy (IVBT), first introduced in 1994, alters vascular biology by slowing neointimal formation and promoting vessel remodeling. These biologic effects frequently persist for up to 2 years.3 This is achieved by free radical formation that damages critical components of the cell cycle to induce apoptosis. IVBT was approved for use in the United States following positive results from several randomized trials focused on bare-metal stent (BMS) restenosis.4,5 However, its popularity declined after studies showed lower restenosis rates with first-generation drug-eluting stents (DES).6,7 While randomized controlled trials comparing IVBT with other therapies for the treatment of recurrent DES ISR are lacking, a retrospective cohort study reported lower rates of major adverse cardiac events (MACE) at 8 months compared with repeat DES treatment for DES ISR. Additionally, several prospective studies have reported better MACE rates for IVBT compared with non-IVBT treatments (including but not limited to repeat DES) for recurrent DES ISR at 1 year.8,9
There are no significant reports detailing long-term follow-up of patients treated with IVBT in the recent years. Available literature includes a 2021 meta-analysis by Megaly et al, which demonstrated that treating recurrent DES ISR with IVBT resulted in a 29.2% incidence of target vessel revascularization over 2 years of follow-up.10 Comparatively, studies demonstrated that restenosis rates with the use of a second layer of DES ranged from 10% to 20%, while balloon angioplasty alone showed a rate 44.6% at 9 to 12 months of follow-up.11 Notably, many retrospective studies have identified a late catch-up phenomenon occurring 12 to 24 months after treatment of recurrent ISR with IVBT, where initially low failure rates significantly increase over time.10,11
This study aimed to evaluate the clinical outcomes of IVBT as a treatment modality for multilayer DES ISR.
Methods
Study population
This study involved patients selected from an observational, single health-system registry including 2 tertiary care centers between 2019 and 2023; the study was approved by the institutional review board. The analysis included patients with anginal symptoms or ischemia who underwent treatment for recurrent DES restenosis using IVBT. Patients presenting with acute stent thrombosis or cardiogenic shock were excluded. For patients who underwent multiple procedures meeting the study criteria, only the index procedure and subsequent staged IVBT procedure were included in the analysis. Baseline demographic data, clinical characteristics, and procedural details were extracted from the registry using standardized electronic entry forms, with data entered by trained cardiology fellows or research staff. Our study was approved by the Northwell Health Institutional Review Board (IRB) with a waiver for informed consent.
Procedure
All patients were either on standard dual antiplatelet therapy (DAPT) prior to the procedure or received loading doses of DAPT before the intervention. Intraprocedural anticoagulation was left to the primary operator but standard dose therapeutic heparin or bivalirudin was administered and monitored per guideline-recommended protocol. Arterial access was obtained via the femoral or radial artery; the choice of access was determined by the primary operator’s discretion.
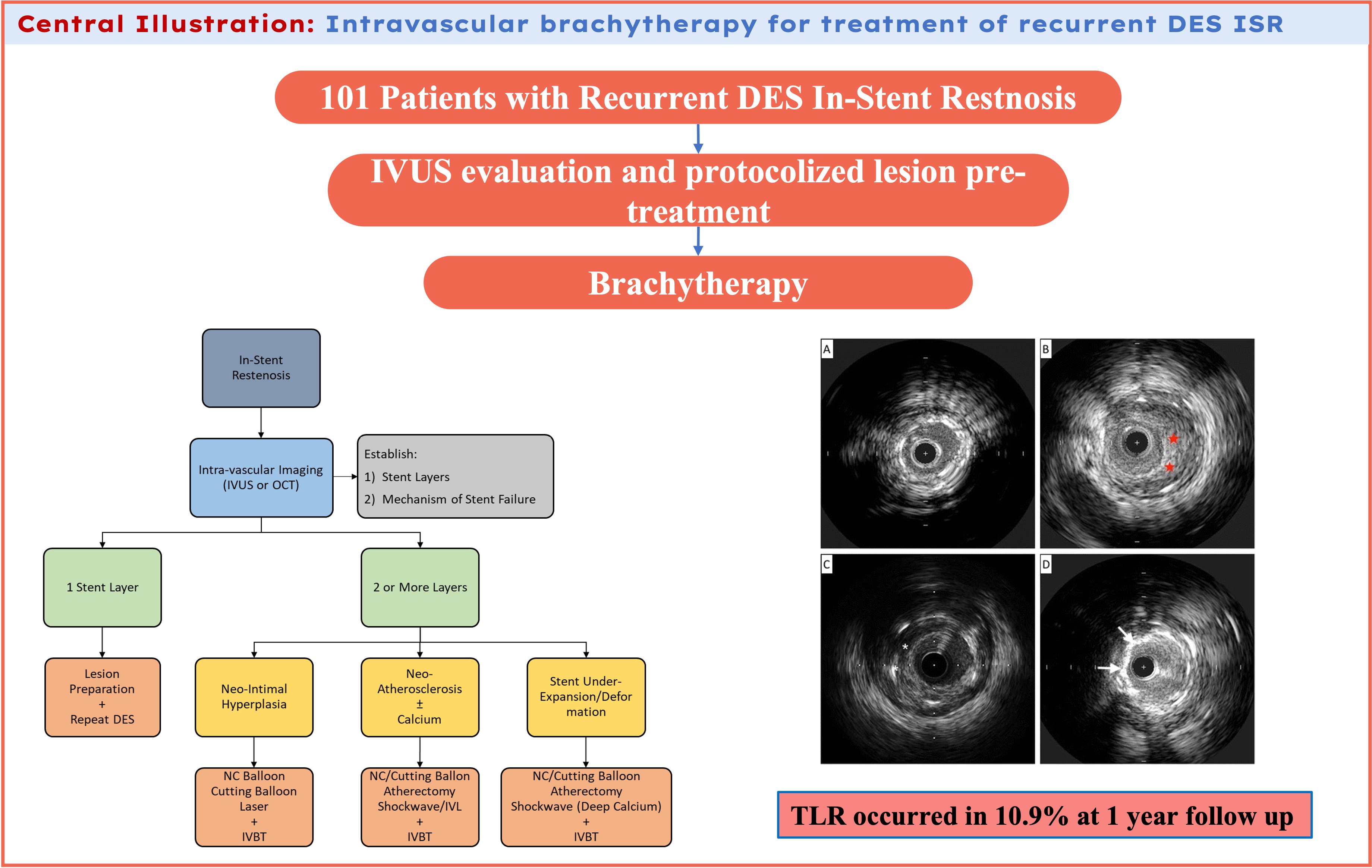
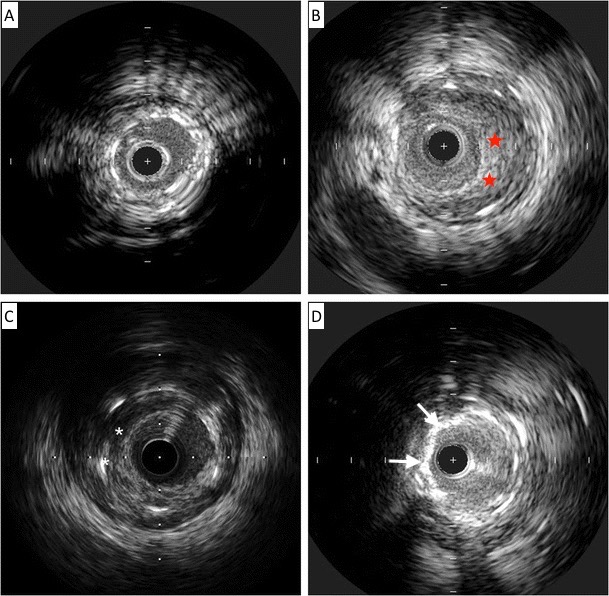
Lesion preparation strategy was determined by the operator and included balloon angioplasty with either non-compliant or cutting balloons, laser atherectomy, or intravascular lithotripsy (IVL), or any combination of methods. Lesion debulking with laser atherectomy was typically performed if balloon angioplasty alone was insufficient to achieve adequate luminal gain. Intravascular ultrasound (IVUS) was the imaging modality of choice and was used at the operator's discretion for lesion and prior stent analysis (Figure 1). Following assessment of the mechanism of ISR and lesion pretreatment, IVBT was delivered using the 40.0-mm Novoste Beta-Cath system (Best Vascular). The prescribed radiation dose was determined by an on-site radiation oncologist who remained present during the entirety of the procedure. A margin of 10.0 mm was maintained at both ends of the source to ensure adequate coverage of the lesion edges, with overlapping dwell times employed for longer lesions. Post-radiation stenting was avoided unless deemed necessary to achieve technical success or complication management. All patients were maintained on DAPT following the procedure for at least 1 year.
Clinical endpoints
The primary outcome assessed was target lesion revascularization (TLR) at 1 year. Secondary endpoints were aimed to establish procedural safety, namely, peri-procedural MACE, vascular complications, and 30-day readmission. MACE was defined as the occurrence of mortality, periprocedural myocardial infarction (MI), need for urgent revascularization, or coronary artery perforation.
Follow-up
Follow-up data was obtained either through hospital visits or telephone contact by dedicated research staff at 30 days and 1 year post-procedure.
Results
Baseline characteristics
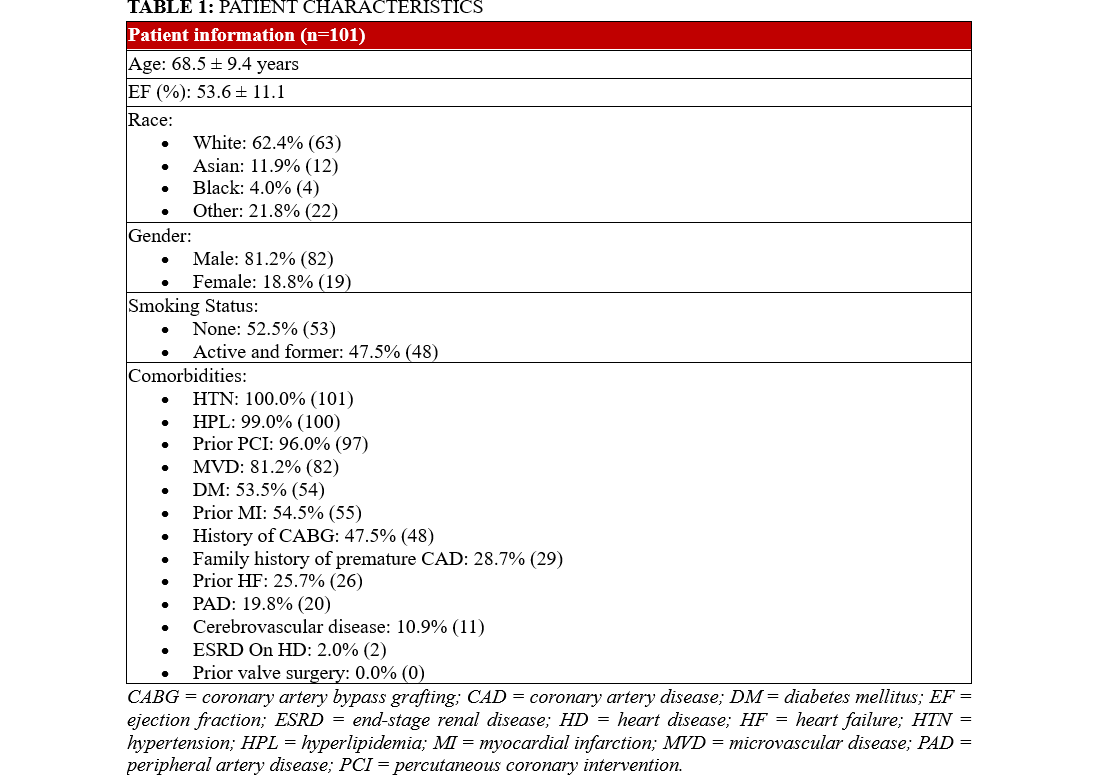
The patient population in the study was 81% (82) male, and 54% (54) had type 2 diabetes mellitus, 100% (101) had hypertension, and 99% (100) had hyperlipidemia. The mean age in this sample was 68.6 years. Of the sample, 23% (23) had advanced chronic kidney disease (CKD) defined as CKD Stage 3b, Stage 4, or end-stage renal disease requiring hemodialysis. The remainder of patient characteristics can be seen in Table 1.
Lesion characteristics/procedural data
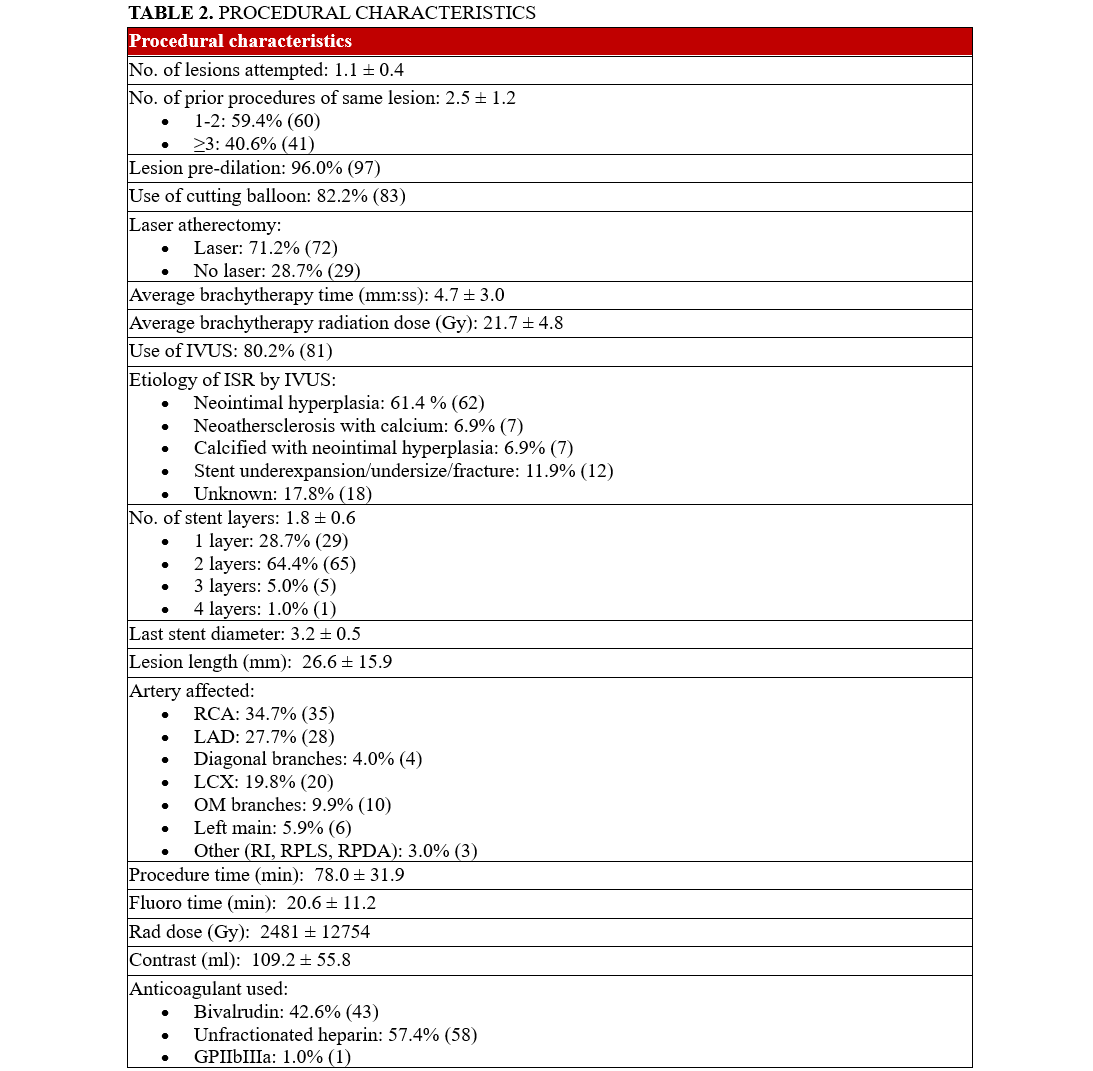
Table 2 demonstrates the details of lesion characteristics and procedural data. The lesions in which interventions took place had a mean stent layer of 1.8; 70.3% (71) of the lesions had 2 or more stent layers (Table 2). The average ISR lesion length was 26.6 mm. Neointimal hyperplasia (NIH) was the primary mechanism for ISR in 61.4% (62) of the lesions. Neoatherosclerosis and stent underexpansion were present in 6.9% (7) and 11.9% (12) of the lesions, respectively. All ISR lesions were pretreated with either balloon angioplasty (82.2% [83]) or laser atherectomy (71.2% [72]). A small percentage of lesions (19.8% [20]) were prepped with IVL for either calcified neoatherosclerosis or medial calcifications which was prohibiting the original stent expansion. The average IVBT dwell time was 5 minutes and 40 seconds with a mean radiation dose of 21.81 Gy.
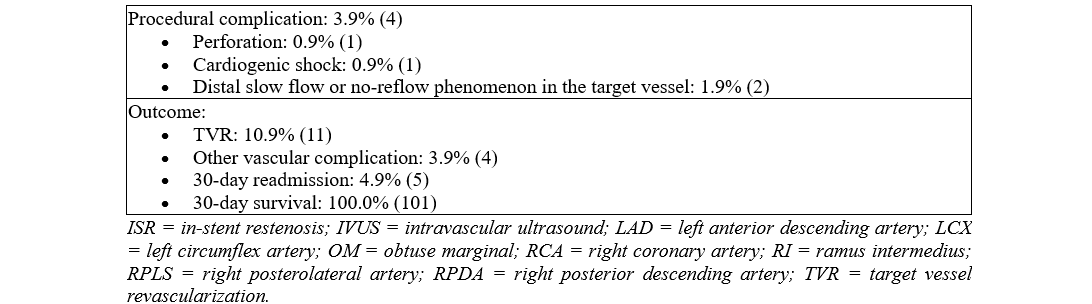
Outcomes
Of the 101 patients analyzed, 10.9% (11) of the patients underwent TLR during the 12-month follow-up period. In terms of patient safety and secondary outcomes, 4.9% (5) of patients were readmitted because of angina within 30 days post-procedure. Vascular complications, defined as Bleeding Academic Research Consortium (BARC) type 2 related to femoral access site issues, occurred in 3.9% (4) of the patients. MACE was limited to 0.9% (1) of the patients; the case resulted from a procedural complication of coronary artery perforation. Notably, there were no instances of peri-procedural MIs, urgent need for revascularization within 24 hours, need for mechanical support, nor cardiac arrest/need for cardiopulmonary resuscitation.
Discussion
This study represents a large series reporting durable 1-year outcomes of IVBT in patients with recurrent DES ISR, including a significant proportion of cases with both multilayer and long segment ISR. Our 1-year follow-up is similar to other contemporary cohort analyses comparing short-term outcomes of IVBT. The key findings of our study include the safety of IVBT for recurrent DES ISR, and the 1-year durability of treatment with a TLR rate of roughly 10%.
IVBT was initially approved based on data from studies of bare-metal stent (BMS) restenosis, although randomized data specific to DES restenosis are lacking. Negi et al demonstrated the safety and efficacy of IVBT for recurrent DES ISR in a single-center retrospective study, while Mangione et al reported a 1-year TLR rate of 24% following IVBT for recurrent DES ISR.12,13 More contemporary data has examined the long-term outcomes of IVBT for recurrent DES ISR, reporting target lesion failure (TLF) rates of 18% at 1 year, which dramatically increased to 44% at 3 years because of the described late catch-up phenomenon.14-16
The IVBT failure rates observed in our study were consistent, if not slightly improved, when compared with existing literature, with a TLR rate of 10.9% at 1 year. This durability at the 1-year follow-up is encouraging given the high prevalence of multilayer ISR and long-average segment of disease, all in a high comorbid patient population. Additionally, our group demonstrates a diligent approach to IVUS-guided pretreatment with an effort to optimize both luminal area and IVBT delivery, as we hypothesize that adequate ISR preparation may be the contributing factor to slightly lower TLR in our cohort compared with the existing literature. The lesion preparation algorithm is depicted in Figure 2.
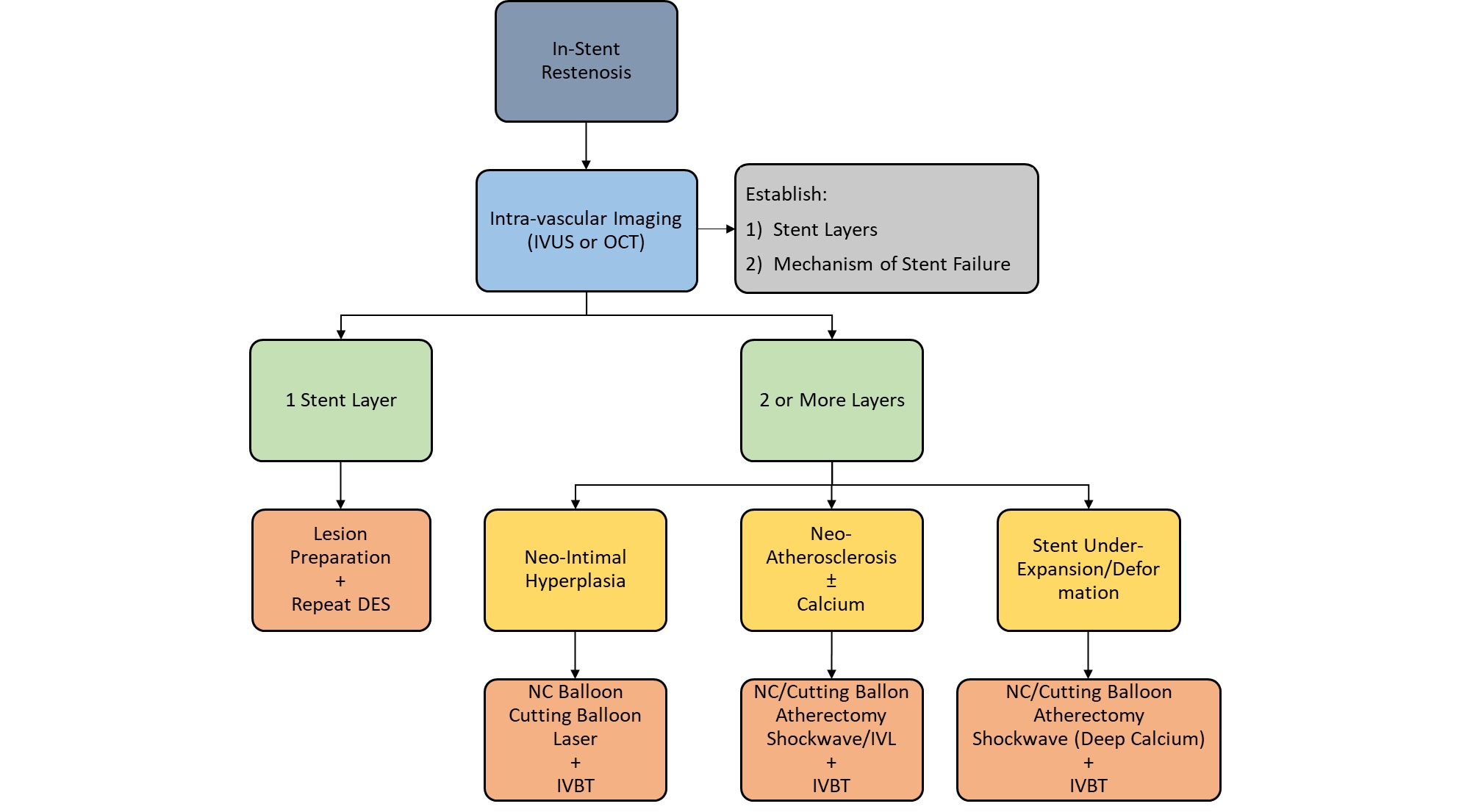
It is worth noting that while numerous factors for ISR have been identified (multilayer stents, stent underexpansion, small vessel size, etc), there remains a limited understanding of the factors contributing to IVBT failure in recurrent ISR.14 The late catch-up phenomenon of IVBT is well described, but, in previous studies, there appears to be no correlation between TLF and the method of disease pretreatment or the use of intravascular imaging. In previous analyses, the strongest predictors for TLF and TLR are increasing stent layers, with the highest rate of TLF at 1 and 3 years in patients with 3 layers of stents. This suggests that TLF and TLR are not entirely a mechanical phenomenon; rather, multiple restenosis events likely indicate an underlying adverse biological substrate. Additionally, it is possible that vascular inflammation, induced by aggressive lesion modification or debulking prior to irradiation, may provoke a proliferative response that negates the luminal patency achieved. The particularly poor outcomes observed in cases with 3 stent layers suggest that IVBT should be considered before a third stent layer is placed. These findings also indicate potential value in exploring the use of IVBT as a treatment option during the initial restenosis event, warranting further investigation.
This study builds upon existing technologies for the management of recurrent DES ISR. A notable innovation recently introduced in the United States is the use of drug-coated balloons (DCB) for the treatment of DES ISR. DCBs deliver an anti-proliferative agent to inhibit NIH progression following adequate lesion preparation. However, current research on this modality has been limited to single-layer ISR, with comparisons restricted to balloon angioplasty. Consequently, the efficacy and long-term outcomes of DCBs in the context of multilayer ISR with diverse underlying mechanisms—such as posterior wall calcification, neoatherosclerosis, NIH, or their combination—remain inadequately defined. We propose a hybrid approach for the future management of multilayer ISR, integrating intravascular imaging-guided lesion preparation, followed by sequential DCB therapy and brachytherapy tailored to ISR characteristics. Further investigations are warranted to assess the utility of combination or staggered therapies, as well as their long-term clinical outcomes.
Limitations
Our study is limited by the retrospective nature of evaluation and the relatively short follow-up of 1 year; however it is hypothesis-generating for the routine use of IVBT in this commonly encountered clinical scenario. Larger and prospective randomized studies are needed.
Conclusions
Recurrent DES ISR continues to present a therapeutic challenge. This study highlights IVBT as a safe and feasible treatment option with durable outcomes at 1-year follow-up. We hypothesize that aggressive lesion debulking followed by IVBT was crucial to maintain a TLR rate lower than previously reported in the existing literature. Large and prospective randomized studies are needed to confirm these findings. Additional research should be performed to determine if repeated IVBT cycles or dose-specific radiations effects aid in preventing recurrent DES ISR. In our study, IVBT proves to be a safe and effective treatment modality for multilayer ISR.
Affiliations and Disclosures
Atul D. Bali, MD1,2; Sandrine Lebrun, MD1,2; Abduljabar Adi, MD1; Varinder Singh, MD1,2; Michael C. Kim, MD1,2; Arber Kodra, MD1,2
From 1Northwell Health at Lenox Hill Hospital, New York, New York; 2Donald and Barbara Zucker School of Medicine at Hofstra/Northwell, Uniondale, New York.
Disclosures: The authors report no financial relationships or conflicts of interest regarding the content herein.
Address for correspondence: Atul D. Bali, MD, Department of Cardiology, Lenox Hill Hospital, 130 East 77th Street, 9 Blackhall, New York, NY 10075, USA. Email: abali@northwell.edu
References
- Alfonso F, Coughlan JJ, Giacoppo D, Kastrati A, Byrne RA. Management of in-stent restenosis. EuroIntervention. 2022;18(2):e103-e123. doi:10.4244/EIJ-D-21-01034
- Shlofmitz E, Iantorno M, Waksman R. Restenosis of drug-eluting stents: a new classification system based on disease mechanism to guide treatment and state-of-the-art review. Circ Cardiovasc Interv. 2019;12(8):e007023. doi:10.1161/CIRCINTERVENTIONS.118.007023
- Sabaté M. Secondary revascularization following intracoronary brachytherapy. EuroIntervention. 2009;5 Suppl D:D121-D126.
- Waksman R, Ajani AE, White RL, et al. Five-year follow-up after intracoronary gamma radiation therapy for in-stent restenosis. Circulation. 2004;109(3):340-344. doi:10.1161/01.CIR.0000109488.62415.01
- Urban P, Serruys P, Baumgart D, et al. A multicentre European registry of intraluminal coronary beta brachytherapy. Eur Heart J. 2003;24(7):604-612. doi:10.1016/s0195-668x(02)00617-6
- Salihu A, Roguelov C, Fournier S, Coucke P, Eeckhout E. Intracoronary brachytherapy for restenosis: 20 years of follow-up. Cardiovasc Revasc Med. 2023;54:1-4. doi:10.1016/j.carrev.2023.04.009
- Stone GW, Ellis SG, O'Shaughnessy CD, et al. Paclitaxel-eluting stents vs vascular brachytherapy for in-stent restenosis within bare-metal stents: the TAXUS V ISR randomized trial. JAMA. 2006;295(11):1253-1263. doi:10.1001/jama.295.11.1253
- Ohri N, Sharma S, Kini A, et al. Intracoronary brachytherapy for in-stent restenosis of drug-eluting stents. Adv Radiat Oncol. 2015;1(1):4-9. doi:10.1016/j.adro.2015.12.002
- Torguson R, Sabaté M, Deible R, et al. Intravascular brachytherapy versus drug-eluting stents for the treatment of patients with drug-eluting stent restenosis. Am J Cardiol. 2006;98(10):1340-1344. doi:10.1016/j.amjcard.2006.06.027
- Megaly M, Glogoza M, Xenogiannis I, et al. Outcomes of intravascular brachytherapy for recurrent drug-eluting in-stent restenosis. Catheter Cardiovasc Interv. 2021;97(1):32-38. doi:10.1002/ccd.28716
- Her AY, Shin ES. Current management of in-stent restenosis. Korean Circ J. 2018;48(5):337-349. doi:10.4070/kcj.2018.0103
- Negi SI, Torguson R, Gai J, et al. Intracoronary brachytherapy for recurrent drug-eluting stent failure. JACC Cardiovasc Interv. 2016;9(12):1259-1265. doi:10.1016/j.jcin.2016.03.018
- Mangione FM, Jatene T, Badr Eslam R, et al. Usefulness of intracoronary brachytherapy for patients with resistant drug-eluting stent restenosis. Am J Cardiol. 2017;120(3):369-373. doi:10.1016/j.amjcard.2017.04.036
- Ho E, Denby K, Cherian S, et al. Intracoronary brachytherapy for drug-eluting stent restenosis: outcomes and clinical correlates. J Soc Cardiovasc Angiogr Interv. 2023;2(1):100550. doi:10.1016/j.jscai.2022.100550
- Megaly M, Glogoza M, Xenogiannis I, et al. Coronary intravascular brachytherapy for recurrent coronary drug-eluting stent in-stent restenosis: a systematic review and meta-analysis. Cardiovasc Revasc Med. 2021;23:28-35. doi:10.1016/j.carrev.2020.08.035
- Negi SI, Torguson R, Gai J, et al. Intracoronary brachytherapy for recurrent drug-eluting stent failure. JACC Cardiovasc Interv. 2016;9(12):1259-1265. doi:10.1016/j.jcin.2016.03.018





















