Complications Associated With Use of the Wolverine Cutting Balloon: Insights From the Manufacturer and User Facility Device Experience (MAUDE) Database
© 2025 HMP Global. All Rights Reserved.
Any views and opinions expressed are those of the author(s) and/or participants and do not necessarily reflect the views, policy, or position of the Journal of Invasive Cardiology or HMP Global, their employees, and affiliates.
Abstract
Objectives. The Wolverine cutting balloon (CB) (Boston Scientific) is a specialized balloon catheter with microsurgical blades that is used for balloon-resistant lesions. The Manufacturer and User Facility Device Experience (MAUDE) database serves as a repository for reports of medical device complications. The aim of this study was to analyze complications associated with CB use during percutaneous coronary intervention in real-world contemporary practice.
Methods. The MAUDE database was searched from January 1, 2020, through December 31, 2023 for reports of complications associated with CB use. Data from individual reports were extracted for analysis.
Results. The final analysis included 2278 complications, of which 97.3% (n = 2216) were associated with device malfunction, 2.4% (n = 55) were associated with patient injury, and 0.3% (n = 7) were associated with patient death. The most common complication overall was balloon rupture (n = 1847), while the most common complication associated with patient injury or death was device entrapment (n = 33). The median number of inflations was significantly higher for complications associated with patient injury or death (3 [IQR 1-8]) compared with complications associated with device malfunction (1[IQR 1-2]) (P = .0035). The median maximum inflation pressure was significantly higher for complications associated with patient injury or death (12 [IQR 11-15] atm) compared with complications associated with device malfunction (8 [IQR 6-10] atm) (P = .0001).
Conclusions. Overall, 2.7% of reported complications were associated with patient injury or death. The most common complication overall was balloon rupture. Device entrapment was the most common complication among reports associated with patient injury or death. Higher inflation pressures and greater number of inflations may be associated with adverse outcomes.
Introduction
Adequate lesion preparation before stent implantation is imperative for achieving favorable outcomes after percutaneous coronary intervention (PCI). Failure to achieve optimal lesion modification can lead to stent underexpansion and subsequent increased risk of in-stent restenosis and stent thrombosis.1 The Wolverine cutting balloon (CB) (Boston Scientific) is a specialized balloon catheter with 3 to 4 microsurgical blades longitudinally bonded on the balloon surface and is indicated for use in balloon-resistant lesions. It is available in diameters ranging from 2.00 to 4.00 mm and in lengths of 6, 10, or 15 mm. This unique design allows for anchoring in tissue and focal application of force to create precise longitudinal incisions in resistant fibrotic and calcific lesions.2,3 Recent trials have demonstrated the efficacy of CB use for the preparation of calcified lesions during PCI, with relatively low complication rates.4-6
The Manufacturer and User Facility Device Experience (MAUDE) database is a repository of medical device reports that is maintained by the Food and Drug Administration (FDA) for post-market surveillance. The reports can be submitted by mandatory reporters including manufacturers and user facilities (eg, hospitals, ambulatory surgical centers) or voluntary reporters (eg, clinicians). The MAUDE database can provide insights about device complications that occur during routine clinical practice, outside the setting of clinical trials.7 The purpose of this study was to explore and analyze complications reported in the MAUDE database that are associated with the use of CBs during PCI.
Methods
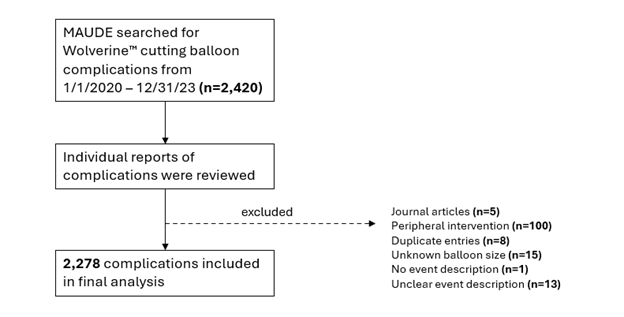
The MAUDE database was searched over a 4-year period from January 1, 2020, through December 31, 2023 for complications involving CBs. The initial search yielded a total of 2420 reported complications. After excluding reports that were duplicates (n = 8), pertained to peripheral interventions (n = 100), originated from journal articles (n = 5), did not provide a balloon size (n = 15), did not provide an event description (n = 1) or had an unclear event description (n = 13), a total of 2278 reports were included in the final analysis (Figure 1). Data elements that were extracted for analysis and were available in all reports included date received, event type (device malfunction, patient injury, or patient death), and balloon diameter. Data elements that were available in only some reports included affected vessel, severity of tortuosity, severity of calcification, number of inflations, and maximum inflation pressure. Data collection was performed by AL, DJ, MA, JN, FK, and SM. The MAUDE database is publicly available at https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfmaude/search.cfm and is comprised of medical device reports that are de-identified. Therefore, institutional review board approval was not required for this study.
Statistical analysis
Categorical variables are expressed as absolute numbers and percentages. Percentages were derived using the total number of reported complications as the denominator since the total number of CBs used in clinical practice over the study period could not be ascertained from the MAUDE database. Continuous variables are expressed as median and interquartile range. Comparison of categorical variables was performed using the Fisher’s exact test. Comparison of continuous variables between groups was performed using the Kruskal-Wallis test. Results were considered significant if the P-value was less than 0.05. All data analysis was performed using StataMP 18 (StataCorp) and R 4.4.2 (The R Foundation).
Results
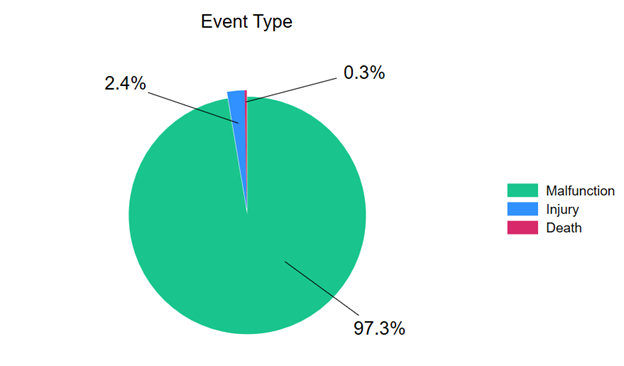
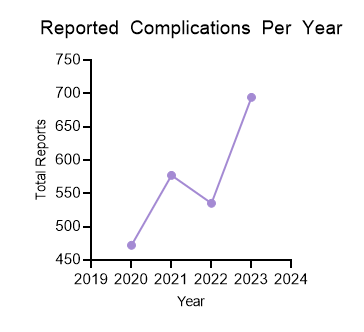
Of the 2278 reported complications associated with CBs included in the analysis, 97.3% (n = 2216) were associated with device malfunction, 2.4% (n = 55) were associated with patient injury, and 0.3% (n = 7) were associated with patient death (Figure 2). The number of reported complications was lowest in 2020 and highest in 2023 (Figure 3), perhaps reflecting the decrease in PCI that occurred during the COVID-19 pandemic. 8,9
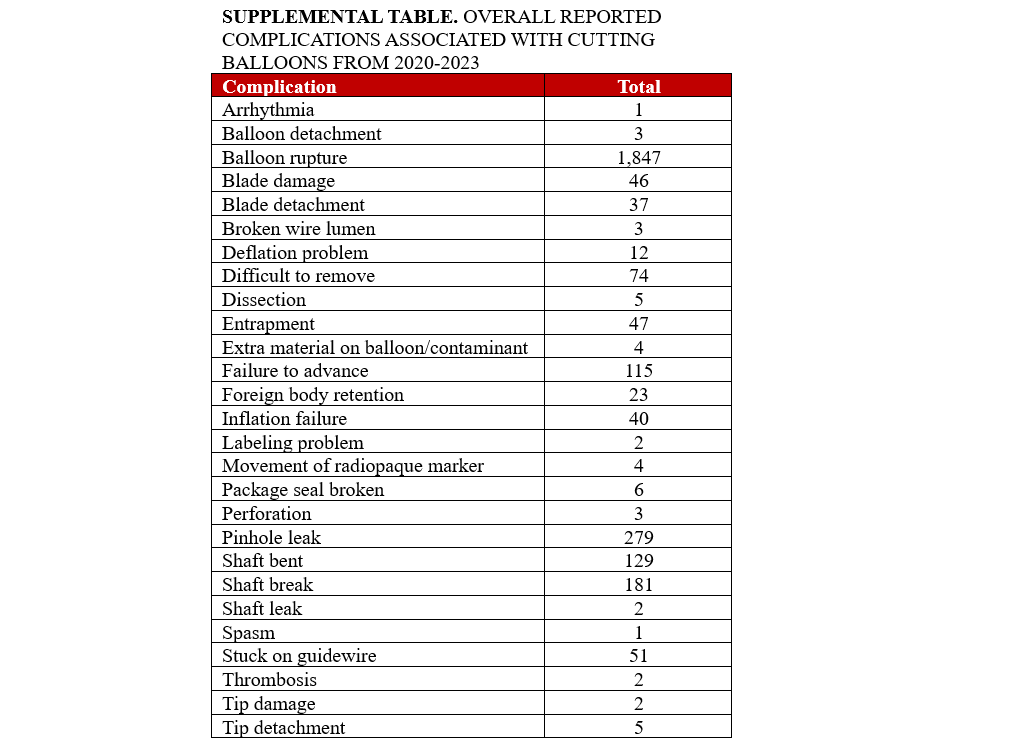
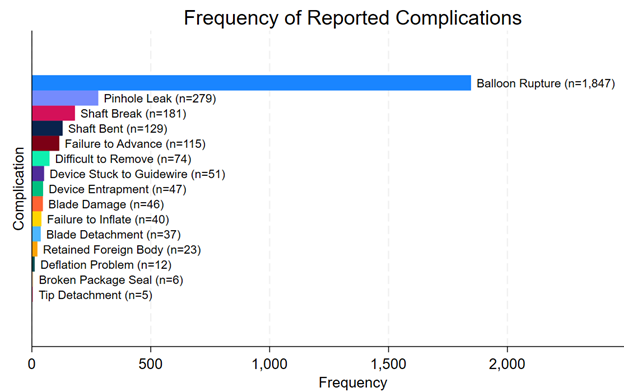
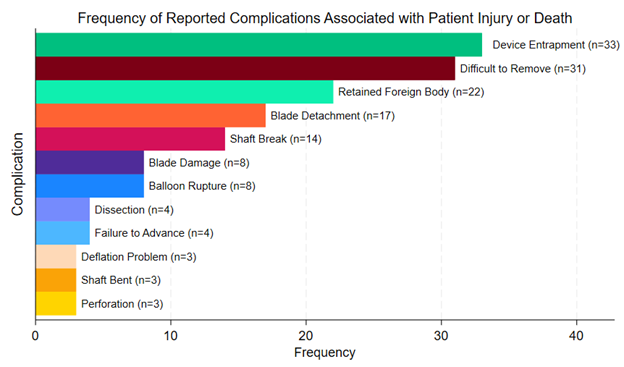
The most frequently reported complication overall was overwhelmingly balloon rupture (n = 1847, 81%), followed by pinhole leak (n = 279, 12%), shaft break (n = 181, 7.9%), shaft bend (n = 129, 5.7%), failure to advance (n = 115, 5.0%), difficult to remove (n = 74, 3.2%), device stuck to guidewire (n = 51, 2.2%), device entrapment (n = 47, 2.1%), and blade damage (n = 46, 2.0%) (Figure 4). A full list of reported complications is provided in the Supplemental Table. Among complications that resulted in patient injury or death, the most common complications were device entrapment (n = 33, 53%), difficult to remove (n = 31, 50%), retained foreign body (n = 22, 35%), blade detachment (n= 17, 27%), shaft break (n = 14, 23%), blade damage (n = 8, 13%), and balloon rupture (n = 8, 13%) (Figure 5). Among complications that resulted in patient death, 3 involved device entrapment, 2 involved dissection, 1 involved perforation, and 1 involved balloon rupture.
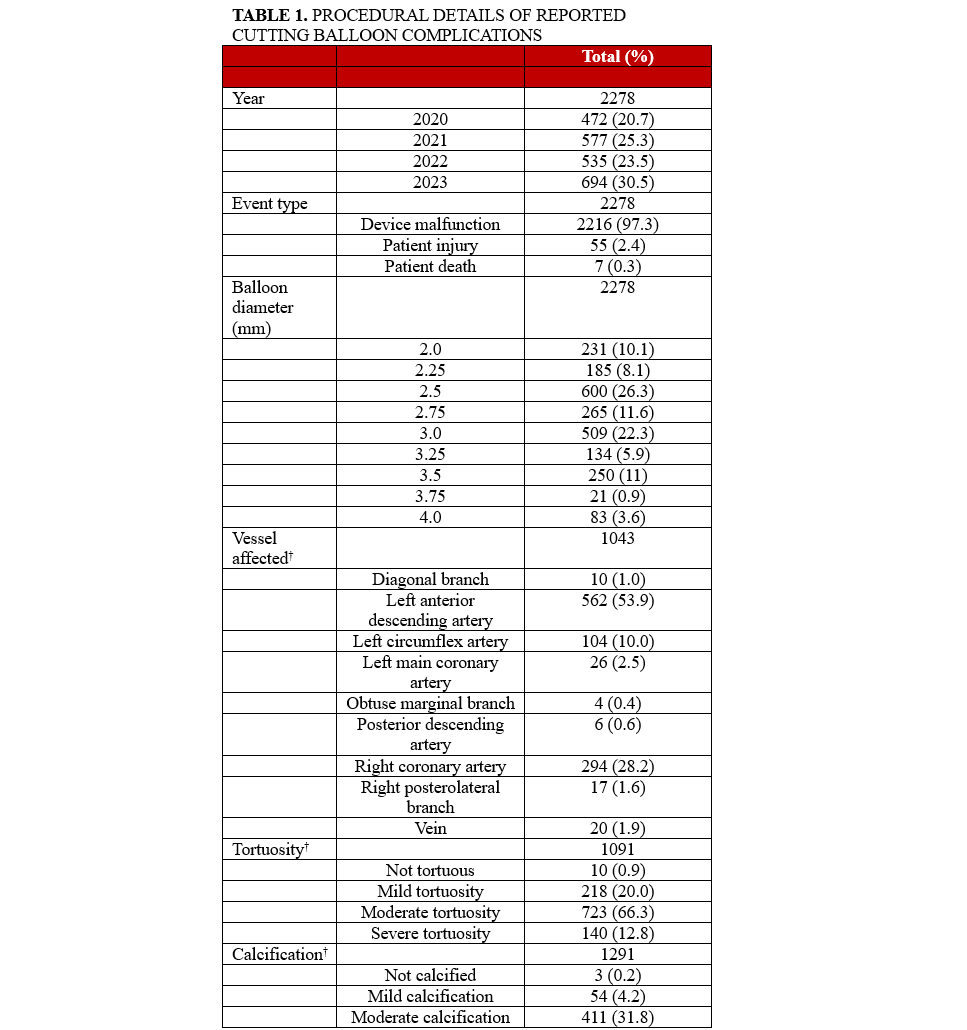
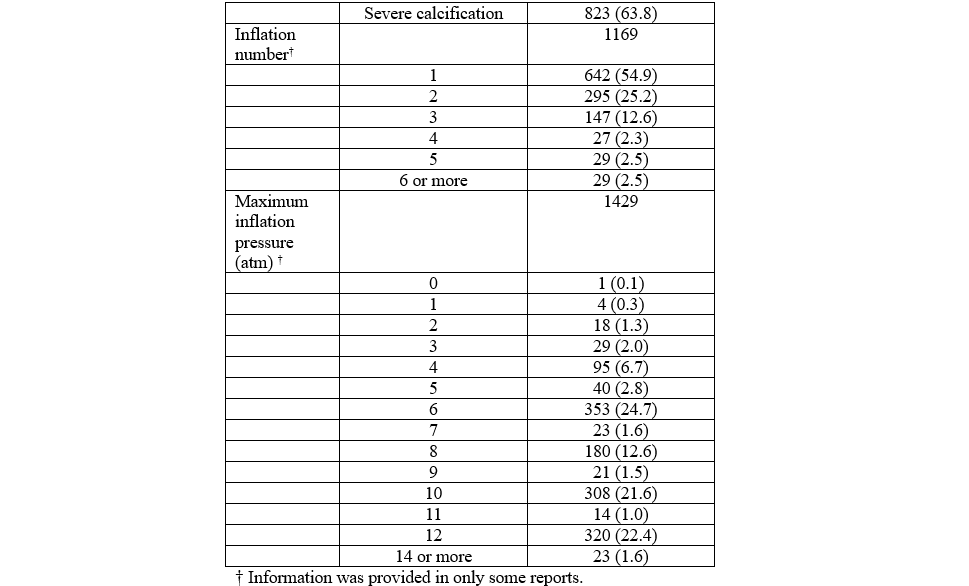
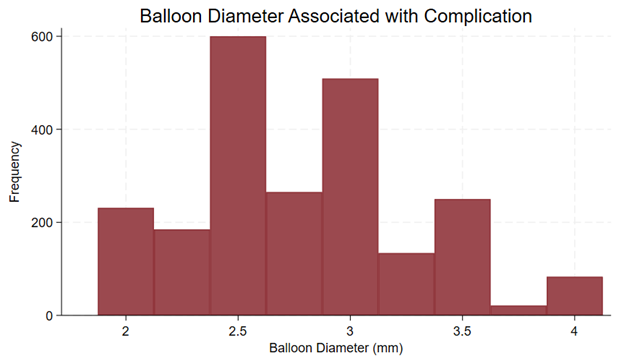
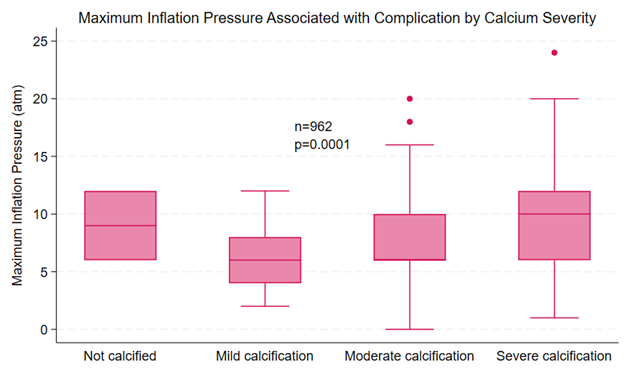
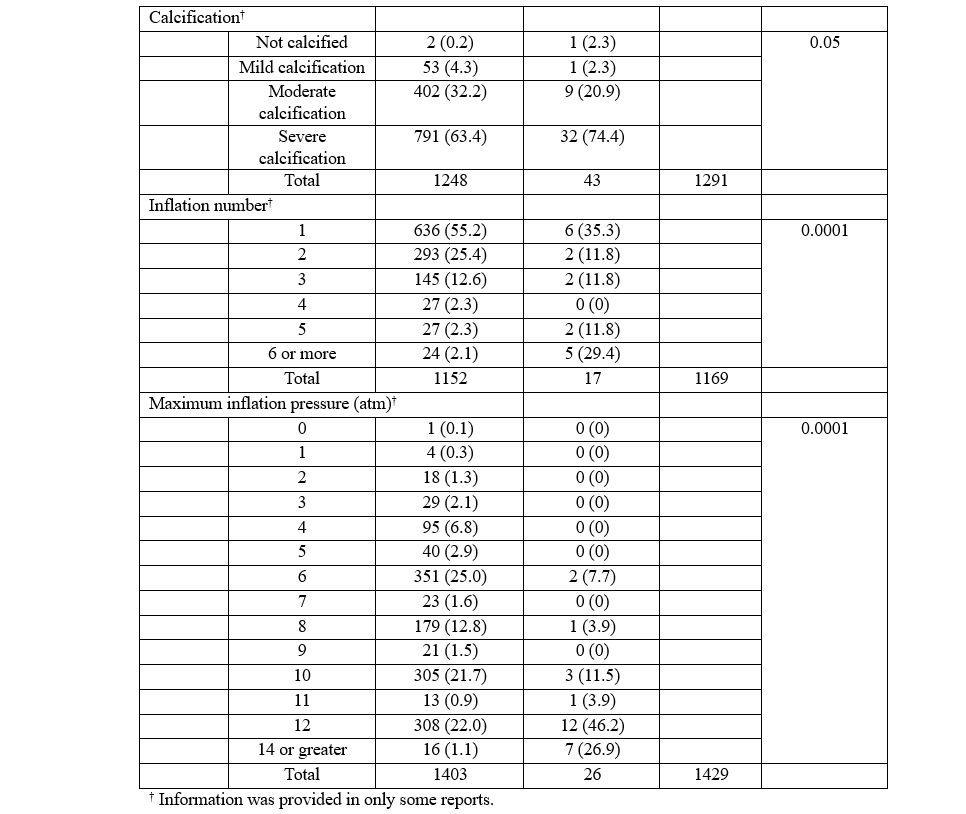
Procedural details provided in the reports of complications are summarized in Table 1. All analyzed reports included balloon diameter. The most common balloon diameter associated with complications was 2.5 mm (n = 600, 26%), followed by 3.0 mm (n = 509, 22%) and 2.75 mm (n = 265, 12%) (Figure 6). The affected vessel was specified in 1043 reports. Of these reports, 562 (53.9%) involved the left anterior descending artery, 294 (28.2%) involved the right coronary artery, and 104 (10%) involved the left circumflex artery. The inflation pressure was provided in 1429 reports. The median inflation pressure was 8 (IQR 6-10) atm. The number of balloon inflations was provided in 1169 reports. The median number of balloon inflations was 1 (IQR 1-2). The severity of calcification was provided in 1291 reports. The target vessel was not calcified in 3 reports, mildly calcified in 54 reports, moderately calcified in 411 reports, and severely calcified in 823 reports. The severity of tortuosity was provided in 1091 reports. The target vessel was not tortuous in 10 reports, mildly tortuous in 218 reports, moderately tortuous in 723 reports, and severely tortuous in 140 reports. Both inflation pressure and calcium severity were provided in 962 reports. The median inflation pressure was 9 (IQR 6-12) atm for non-calcified vessels (n = 2), 6 (IQR 4-8) atm for mildly calcified vessels (n = 40), 6 (IQR 6-10) atm for moderately calcified vessels (n = 306), and 10 (IQR 6-12) atm for severely calcified vessels (n = 614) (P = .0001) (Figure 7).
Complications that resulted in device malfunction were compared to complications that resulted in patient injury or death. While year of complication (P = .464), balloon diameter (P = .273), vessel tortuosity (P = .055), and vessel calcification (P = .05) were not significantly different between the 2 groups, there were significant differences in the vessel affected (P < .0001), number of inflations (P = .0001), and maximum inflation pressure (P = .0001) (Table 2). Complications resulting in patient injury or death were more likely to involve the left main coronary artery (12.2% vs 2.0%) and the diagonal (8.2% vs 0.6%).
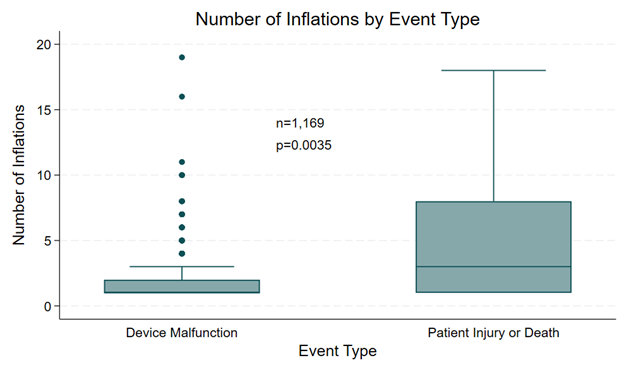
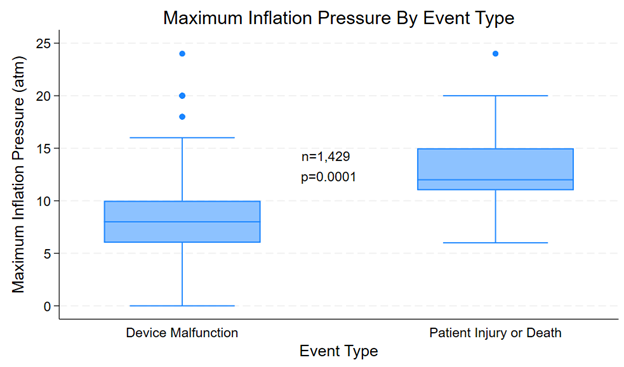
Also, complications resulting in patient injury or death were more likely to be associated with a greater number of inflations; 29.4% of cases resulting in injury or death involved 6 or more inflations, while 2.1% of these cases were associated with device malfunction. The median number of inflations among complications associated with patient injury or death was 3 (IQR 1-8), while the median number of inflations among complications associated with device malfunction was 1 (IQR 1-2) (P = .0035) (Figure 8). Furthermore, complications resulting in patient injury or death were more likely to be associated with higher maximum inflation pressures, with 26.9% involving inflation pressures of 14 atm or greater compared with 1.1% among complications associated with device malfunction. The median maximum inflation pressure among complications associated with patient injury or death was 12 (IQR 11-15) atm, while the median maximum inflation pressure among complications associated with device malfunction was 8 (IQR 6-10) atm (P = .0001) (Figure 9).
Discussion
Early trials of CBs focused on the comparison of cutting balloon angioplasty (CBA) with conventional percutaneous transluminal coronary angioplasty (PTCA) for reducing the rate restenosis. The Global Randomized Trial10 did not show any significant difference in restenosis rates between patients treated with CBA and PTCA for de novo lesions, and the RESCUT trial11 failed to show any benefit of CBA for decreasing the recurrence of in-stent restenosis when compared to PTCA. More recently, there has been increasing use of CBA for the treatment of calcified lesions. In the PREPARE-CALC-COMBO trial, the combination of a CB with rotational atherectomy (RA) during PCI of calcified coronary lesions resulted in higher minimal stent area (MSA) when compared with the use of modified balloon or RA alone.4 In the COPS trial, treatment of calcified coronary lesions with CBs resulted in significantly greater MSA compared with treatment with a noncompliant balloon.5 In contrast, the ROTA-CUT trial showed that treatment of calcified coronary lesions with RA and CBs resulted in similar MSA when compared with a strategy of RA and a noncompliant balloon.6 The complication rates in the CB arms reported in these trials were relatively low, with the rate of vessel perforation ranging from 0% in ROTA-CUT to 4.4% in COPS. The forthcoming multicenter, randomized controlled Short-Cut trial will aim to demonstrate non-inferiority between CBA and intravascular lithotripsy for the treatment of moderately to severely calcified coronary artery lesions either with or without up-front RA.12
To our knowledge, this is the most comprehensive analysis of complications associated with CB use in contemporary practice. Several findings are notable. First, most reported complications were associated with device malfunction, with 2.7% (n = 62) of reported complications being associated with patient injury or death. The most frequently reported complication overall was balloon rupture (n = 1847, 81%). Among complications that resulted in patient injury or death, the most common complication was device entrapment (n = 33, 53%). The most common balloon diameter associated with reported complications in the study was 2.5 mm.
As expected, the median inflation pressure was significantly higher when the target vessel was severely calcified. Complications resulting in patient injury or death were associated with a greater number of balloon inflations (3 [IQR 1-8]) than complications associated with device malfunction (1 [IQR 1-2]). Additionally, we found that the median balloon inflation pressure associated with patient injury or death (12 [IQR 11-15] atm) was significantly higher compared with the median balloon inflation pressure associated with device malfunction (8 [IQR 6-10] atm). This trend aligns with findings from the recent COPS5 and ROTA-CUT6 trials; there were 2 perforations in the COPS trial where the mean CB inflation pressure was 18.3 ± 5 atm, while there were no perforations in the ROTA-CUT trial where the mean CB inflation pressure was 12.3 ± 3.9 atm. It is noteworthy that the manufacturer’s instructions for use indicate that the maximum inflation pressure of 12 atm should not be exceeded.13 With these results in mind, it seems that avoiding inflation pressures that exceed manufacturer recommendations may be associated with improved intraprocedural patient safety. On the other hand, based on the PREPARE-CALC-COMBO, COPS, and ROTA-CUT trials, it seems that higher CB inflation pressures result in greater MSA.14 Given that there have been several prior studies that have demonstrated lower MSA to be a predictor of future adverse events,15 the intraprocedural risks of higher CB inflation pressures must be balanced against the potential long-term benefits of greater MSA. If high inflation pressures are used with a CB, the CB should be downsized by 0.5 mm in relation to the media-to-media diameter on intravascular ultrasound imaging to minimize the risk of coronary artery perforation.5
The reported frequency of perforation in our analysis was low (n = 3, 0.1%). The most common complications that resulted in patient injury or death were device entrapment and difficult device removal. Device entrapment during PCI is a potentially serious complication that can lead to myocardial infarction, vessel thrombosis, or vessel perforation. Balloon entrapment is more likely to occur in cases of balloon rupture, entanglement with a previous stent, or forceful advancement through severely calcified lesions.16 While device retrieval can often be successfully achieved with percutaneous techniques, surgical removal may be required in some cases. A special consideration pertaining to the use of CBs within a prior stent is the possibility of entanglement of the microsurgical blades within stent struts, which subsequently can lead to entrapment17 or even stent avulsion.18 In fact, the manufacturer instructions for use specify that “delivery through the side cell of a previously placed stent” is a contraindication for use, “as the deflated Cutting Balloon could become entangled in the stent.”19 It is important for operators to be aware of the lesion characteristics that increase the risk of device entrapment so that appropriate caution can be exercised to avoid this potentially disastrous complication.
Limitations
There are several limitations to our study that should be noted. As with all studies performed using the MAUDE database, the true incidence of complications cannot be ascertained because the denominator is not known. It should be reiterated that the percentages presented in this manuscript were derived using the total number of reported complications as the denominator. It is also impossible to reliably draw conclusions about causality. Additionally, the level of detail provided in each report was inconsistent and highly variable. Inclusion of more detailed patient and procedural characteristics would allow for more comprehensive analysis and would help to further clarify patterns and trends associated with complications. The database is also prone to underreporting and reporting inaccuracies.20 Therefore, it is important to interpret the results of this study in the context of the limitations of the data source. Given these limitations, the results of this study should be considered exploratory and hypothesis-generating.
Conclusions
Based on device complications associated with CBs as reported in the MAUDE database over a 4-year period from 2020 through 2023, 2.4% of reported complications were associated with patient injury, and 0.3% were associated with patient death. Overall, the vast majority of reported complications (97.3%) were associated with device malfunction, with the most common complication being balloon rupture. Among complications associated with patient injury or death, the most common complication was device entrapment. Balloon inflation pressures were significantly higher with worsening calcium severity. Also, balloon inflation pressures and the total number of inflations were both significantly higher among complications that resulted in patient injury or death compared with complications associated with device malfunction. These findings suggest that higher inflation pressures and the total number of inflations may be associated with an increased risk of adverse outcomes and warrant careful consideration by operators. However, given the limitations of the data source, these results should largely be considered hypothesis-generating.
Affiliations and Disclosures
Aaron Lee, MD1; Dorothy Jung, MD1; Mohammad Ahmed, DO1; Jonathan Norton, DO2; Francois Kaleta, DO3; Samer Muallem, MD4; Maulin Patel, DO1; Moses Mathur, MD1, Mark Kozak, MD1; Ian C. Gilchrist, MD1; Chad J. Zack, MD1
From the 1Penn State Milton S. Hershey Medical Center, Hershey, Pennsylvania; 2WellSpan Good Samaritan Hospital, Lebanon, Pennsylvania; 3Cook County Health, Chicago, Illinois; 4Northwestern Memorial Hospital, Chicago, Illinois.
Disclosures: The authors report no financial relationships or conflicts of interest regarding the content herein.
Address for correspondence: Aaron Lee, MD, FACC, FSCAI, 500 University Drive, P.O. Box 850, Hershey, PA 17033, USA. Email: alee10@pennstatehealth.psu.edu; X: @AaronLeeMD
References
1. Moses JW, Carlier S, Moussa I. Lesion preparation prior to stenting. Rev Cardiovasc Med. 2004;5 Suppl 2:S16-S21.
2. WOLVERINE™ Coronary Cutting Balloon™ Product Details. Boston Scientific. Accessed April 21, 2024. https://www.bostonscientific.com/content/gwc/en-US/products/balloons-cutting/wolverine-cutting-balloon.html
3. Clear-cut control in complex lesions. Boston Scientific. Accessed April 21, 2024. https://www.bostonscientific.com/en-US/medical-specialties/interventional-cardiology/coronary-interventions/pci-product-portfolio/wolverine.html
4. Allali A, Toelg R, Abdel-Wahab M, et al. Combined rotational atherectomy and cutting balloon angioplasty prior to drug-eluting stent implantation in severely calcified coronary lesions: the PREPARE-CALC-COMBO study. Catheter Cardiovasc Interv. 2022;100(6):979-989. doi:10.1002/ccd.30423
5. Mangieri A, Nerla R, Castriota F, et al. Cutting balloon to optimize predilation for stent implantation: the COPS randomized trial. Catheter Cardiovasc Interv. 2023;101(4):798-805. doi:10.1002/ccd.30603
6. Sharma SK, Mehran R, Vogel B, et al. Rotational atherectomy combined with cutting balloon to optimise stent expansion in calcified lesions: the ROTA-CUT randomised trial. EuroIntervention. 2024;20(1):75-84. doi:10.4244/EIJ-D-23-00811
7. Gurtcheff SE. Introduction to the MAUDE database. Clin Obstet Gynecol. 2008;51(1):120-123. doi:10.1097/GRF.0b013e318161e657
8. Azzalini L, Seth M, Sukul D, et al. Trends and outcomes of percutaneous coronary intervention during the COVID-19 pandemic in Michigan. PLoS One. 2022;17(9):e0273638. doi:10.1371/journal.pone.0273638
9. Hannan EL, Zhong Y, Cozzens K, et al. Impact of COVID-19 on percutaneous coronary intervention utilization and mortality in New York. Catheter Cardiovasc Interv. 2023;101(6):980-994. doi:10.1002/ccd.30648
10. Mauri L, Bonan R, Weiner BH, et al. Cutting balloon angioplasty for the prevention of restenosis: results of the Cutting Balloon Global Randomized Trial. Am J Cardiol. 2002;90(10):1079-1083. doi:10.1016/s0002-9149(02)02773-x
11. Albiero R, Silber S, Di Mario C, et al; RESCUT Investigators. Cutting balloon versus conventional balloon angioplasty for the treatment of in-stent restenosis: results of the restenosis cutting balloon evaluation trial (RESCUT). J Am Coll Cardiol. 2004;43(6):943-949. doi:10.1016/j.jacc.2003.09.054
12. Shockwave lithoplasty compared to cutting balloon treatment in calcified coronary disease - a randomized controlled trial (Short-Cut). ClinicalTrials.gov identifier: NCT06089135. Updated December 7, 2023. Accessed August 27, 2024, https://clinicaltrials.gov/study/NCT06089135
13. Sharma SK, Vogel B, Mehran R, Moses JW. Reply: the ROTA-CUT randomised trial: push the button harder. EuroIntervention. 2024;20(4):e269. doi:10.4244/EIJ-D-23-00985
14. Novelli L, Colombo A, Mangieri A. Letter: the ROTA-CUT randomised trial: push the button harder. EuroIntervention. 2024;20(4):e268. doi:10.4244/EIJ-D-23-00937
15. Fujimura T, Matsumura M, Witzenbichler B, et al. Stent expansion indexes to predict clinical outcomes: an IVUS substudy from ADAPT-DES. JACC Cardiovasc Interv. 2021;14(15):1639-1650. doi:10.1016/j.jcin.2021.05.019
16. Sanz-Sánchez J, Mashayekhi K, Agostoni P, et al. Device entrapment during percutaneous coronary intervention. Catheter Cardiovasc Interv. 2022;99(6):1766-1777. doi:10.1002/ccd.30160
17. Giugliano GR, Cox N, Popma J. Cutting balloon entrapment during treatment of in-stent restenosis: an unusual complication and its management. J Invasive Cardiol. 2005;17(3):168-170.
18. Vemula P, Kalavakunta JK, Abela GS, Karve M. A rare and serious unforeseen complication of cutting balloon angioplasty. Case Rep Cardiol. 2014;2014:246784. doi:10.1155/2014/246784
19. WOLVERINE™ cutting balloon micro-surgical dilatation catheter: indications, safety, and warnings. Boston Scientific. Accessed August 29, 2024. https://www.bostonscientific.com/en-US/products/balloons-cutting/wolverine-cutting-balloon/wolverine-indications-safety-and-warnings.html
20. About Manufacturer and User Facility Device Experience (MAUDE) Database. U.S. Food and Drug Administration. Accessed August 29, 2024. https://www.fda.gov/medical-devices/mandatory-reporting-requirements-manufacturers-importers-and-device-user-facilities/about-manufacturer-and-user-facility-device-experience-maude-database