Comparison of Fixed Dose Versus Weight-Adjusted Heparin on the Prevention of Radial Artery Occlusion After Diagnostic Transradial Catheterization
© 2025 HMP Global. All Rights Reserved.
Any views and opinions expressed are those of the author(s) and/or participants and do not necessarily reflect the views, policy, or position of the Journal of Invasive Cardiology or HMP Global, their employees, and affiliates.
Abstract
Objectives. Transradial access (TRA) catheterization has demonstrated significant reductions in procedural complications compared with other access routes. However, radial artery occlusion (RAO) remains a concern, and the optimal dose of heparin to mitigate RAO has yet to be determined. This study aims to address this gap by investigating the impact of weight-adjusted heparin doses on the incidence of RAO in patients undergoing diagnostic transradial catheterization.
Methods. This study is a subanalysis of a multicenter, prospective, randomized trial evaluating heparin dosing strategies in 1494 patients undergoing diagnostic transradial catheterization. All participants received a standard fixed dose of 5000 IU of heparin at the start of the procedure, with additional analyses stratifying patients by weight-adjusted heparin doses. RAO was assessed using Doppler ultrasound within 12 hours post-procedure.
Results. Patients were grouped by weight-adjusted heparin quartiles: less than 58.14 IU/kg (Quartile 1), 58.14 to 65.79 IU/kg (Quartile 2), 65.79 to 74.63 IU/kg (Quartile 3), and greater than 74.63 IU/kg (Quartile 4). The incidence of RAO was similar across groups (2.1%, 2.6%, 2.8%, and 3.0%, respectively; P = .86). Comparisons of extreme dosages (< 50 IU/kg vs > 80 IU/kg) also revealed no significant differences (1.9% vs 2.5%; P = .71). No major bleeding events were reported, and hematoma rates were consistent across groups.
Conclusions. Heparin doses adjusted by weight did not significantly influence the incidence of RAO when a baseline dose of 5000 IU was maintained. These findings reinforce the safety and efficacy of using 5000 IU heparin during diagnostic TRA procedures.
Introduction
Transradial access (TRA) has become the preferred approach for coronary catheterization because of its lower incidence of vascular complications and enhanced patient comfort. This approach has consistently shown reduced rates of bleeding and access site infections compared with transfemoral access. However, a common complication of TRA is radial artery occlusion (RAO), which may compromise future access and limit repeat procedures.1
Heparin plays a crucial role in preventing RAO following transradial catheterization. The administration of heparin during the procedure has been shown to significantly reduce the incidence of RAO. The SPIRIT OF ARTEMIS study demonstrated that a higher dose of heparin (100 IU/kg), compared with a standard dose (50 IU/kg), significantly reduced the rate of RAO from 8.1% to 3.0% without compromising safety, suggesting that high-intensity anticoagulation should be considered during transradial diagnostic procedures.2 Additionally, the American Heart Association's guidelines emphasize the importance of procedural anticoagulation, including the use of unfractionated heparin, in combination with best practices such as maintenance of patent hemostasis, to prevent RAO. The guidelines suggest that the optimal dose of unfractionated heparin is 50 IU/kg for diagnostic catheterization, with alternative anticoagulants like bivalirudin or enoxaparin showing similar efficacy in preventing RAO.3
A systematic review and meta-analysis further support the use of higher doses of heparin, indicating that lower doses are associated with increased RAO.4 The review highlights that high-dose heparin, along with shorter compression times and patent hemostasis, is effective in reducing RAO. Another meta-analysis found that higher doses of unfractionated heparin (eg, 5000 IU) were associated with a reduction in RAO compared with lower doses, although there was significant heterogeneity in the studies reviewed.5
Despite its proven benefits, there remains a lack of consensus on the optimal dose of heparin required to prevent RAO without increasing the risk of bleeding. Some studies suggest that higher doses of heparin may offer additional protection against RAO, while others highlight the risk of bleeding complications, particularly in smaller or lower-weight patients.5,6
This study aims to address this gap by investigating the impact of weight-adjusted heparin doses on the incidence of RAO in patients undergoing diagnostic transradial catheterization. By evaluating varying doses within a multicenter, randomized trial framework, this study seeks to provide evidence-based guidance on the optimal anticoagulation strategy for TRA procedures.
Methods
Study design and population
This study is a subanalysis of the PATENS trial, a prospective, multicenter, randomized, double-blind, placebo-controlled clinical trial designed to evaluate interventions for the prevention of RAO following transradial catheterization.7 For the current subanalysis, 1494 consecutive patients who underwent transradial diagnostic catheterization at 3 high-volume centers were included. The institutional medical ethics committees of all participating hospitals approved the study protocol; all patients provided written informed consent before undergoing procedures.
Patients were eligible if they were 18 years or older and underwent diagnostic or therapeutic coronary catheterization via the transradial route using 5-French (Fr) or 6-Fr sheaths. Exclusion criteria included acute myocardial infarction requiring primary angioplasty, procedural complications such as cardiac arrest or stroke, prior inclusion in the trial, known allergies or intolerance to nitrates, and recent use of nitrates or phosphodiesterase inhibitors.
Randomization and procedures
The original trial included randomization to nitroglycerin or placebo at 2 intervention time points. For this subanalysis, weight-adjusted heparin dosing was retrospectively calculated. Patients were stratified into quartiles based on weight-adjusted heparin doses to explore potential dose-response relationships across different weight categories. This method allowed for a nuanced analysis of the impact of varying doses while maintaining a clear framework for comparison. Dose stratification was based on calculating the weight-adjusted heparin dose for each patient. Since all patients received a fixed dose of 5000 IU, the stratification was achieved by dividing this fixed dose by the patient’s body weight, allowing for categorization into quartiles: Quartile 1 (< 58.14 IU/kg), Quartile 2 (58.14-65.79 IU/kg), Quartile 3 (65.79-74.63 IU/kg), and Quartile 4 (> 74.63 IU/kg). Extreme dosage groups (< 50 IU/kg and > 80 IU/kg) were also analyzed.
Transradial access protocol
Radial artery access was achieved under local anesthesia using a palpatory method without ultrasound guidance. A fixed dose of 5000 IU of unfractionated heparin was administered intra-arterially after sheath placement. Hemostasis was achieved using pneumatic compression devices with minimal pressure and a short compression duration (1-2 hours).8
Outcome assessment
The primary outcome was the incidence of early RAO, defined as the absence of anterograde flow on Doppler ultrasound performed within 12 hours of sheath removal. Secondary outcomes included hematoma rates and major bleeding events, classified according to the Bleeding Academic Research Consortium definitions.9
Doppler ultrasound evaluations were performed using high-frequency vascular transducers by operators blinded to the randomization and treatment groups.
Statistical analysis
Patients were analyzed on an intention-to-treat basis. Continuous variables were reported as mean ± SD and compared using the Student’s t-test or Mann-Whitney U test, as appropriate. Categorical variables were expressed as frequencies and percentages and compared using the chi-square or Fisher’s exact test. Multivariable regression analyses were performed to identify predictors of RAO, including weight-adjusted heparin dosing as a covariate.
Results
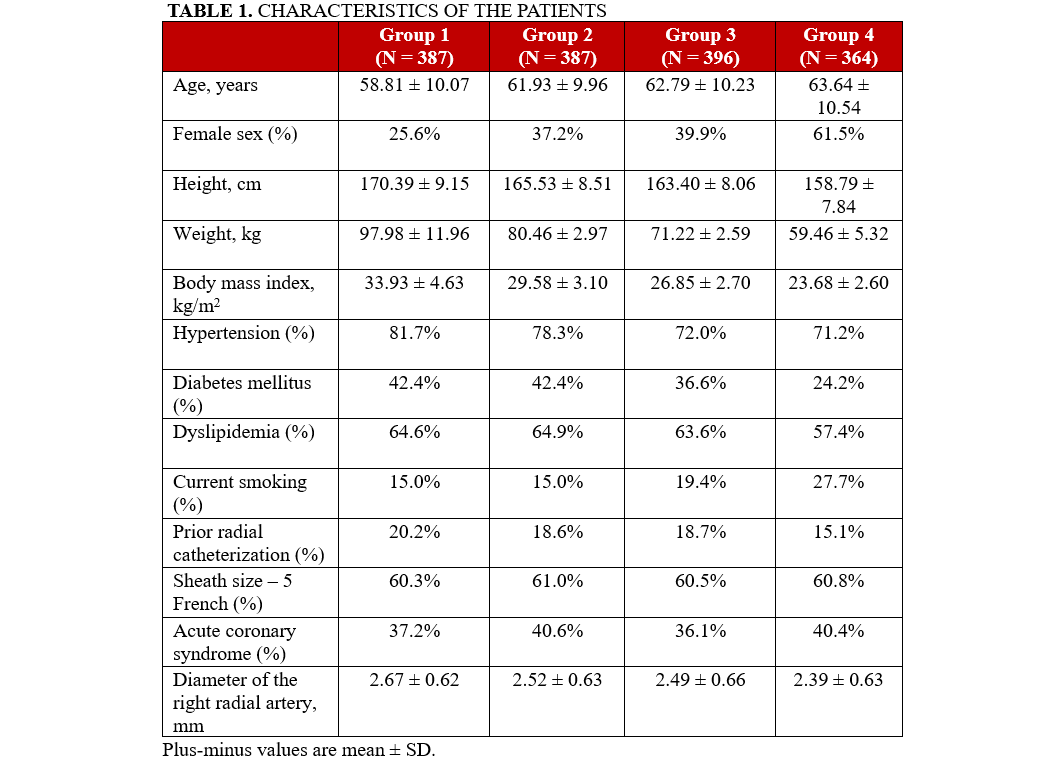
A total of 1494 patients undergoing transradial diagnostic catheterization were analyzed. Patients were categorized into quartiles based on the weight-adjusted heparin dose administered during the procedure. The quartiles were defined as follows: Group 1 (< 58.14 IU/kg), Group 2 (58.14-65.79 IU/kg), Group 3 (65.79-74.63 IU/kg), and Group 4 (> 74.63 IU/kg), comprising 387, 387, 396, and 364 patients, respectively. The baseline characteristics of the groups were largely comparable, apart from variables associated with patient weight, such as gender distribution (Table 1).
Radial artery occlusion incidence
The incidence of RAO, assessed via Doppler ultrasound within the first 12 hours post-procedure, did not differ significantly between the quartile groups: 2.1% (8 patients) in Group 1, 2.6% (10 patients) in Group 2, 2.8% (11 patients) in Group 3, and 3.0% (11 patients) in Group 4 (P = .86) (Table 2).
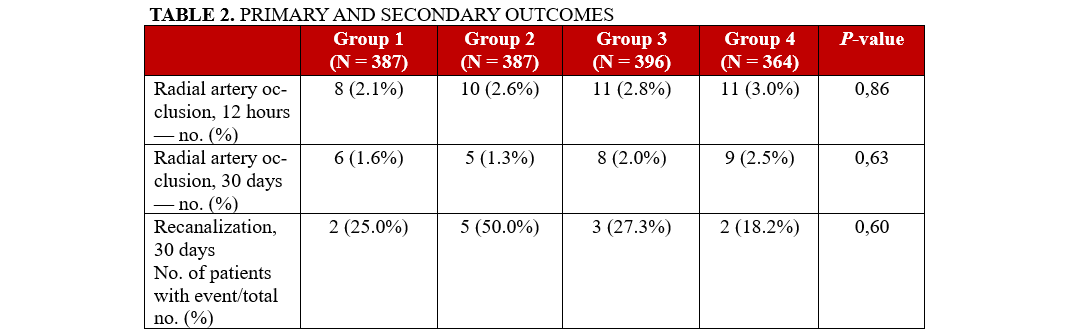
Similarly, when comparing the extreme heparin dose subgroups, defined as less than 50 IU/kg (low-dose) and greater than 80 IU/kg (high-dose), no statistically significant difference in RAO incidence was observed. RAO occurred in 1.9% (5) of patients in the low-dose group and 2.5% (6) of patients in the high-dose group (P = .71). The multivariate analysis showed no significant differences in RAO rates between any independent variable, including the use of 5-Fr and 6-Fr sheath sizes use.
Discussion
Our findings confirm that weight-adjusted heparin dosing does not significantly impact the incidence of RAO when a baseline dose of 5000 IU is administered. This suggests that the standard fixed dose provides a reliable balance between efficacy and safety, potentially streamlining anticoagulation practices in diagnostic TRA procedures.
RAO arises from thrombus formation triggered by mechanical and rheological factors. Key contributors include arterial wall punctures, sheath insertion, catheter manipulation, and local micro-dissections, which collectively lead to endothelial injury. Additionally, compression during hemostasis or hematoma formation induces reduced blood flow and stasis, creating conditions that activate the coagulation cascade as described by Virchow's triad. The use of heparin mitigates these processes by counteracting thrombus formation, thereby reducing the risk of RAO and preserving arterial patency.10
This observation aligns with previous studies that demonstrated a substantial reduction in RAO rates with higher heparin doses, but with diminishing returns above certain thresholds. The SPIRIT OF ARTEMIS trial reported a significant reduction in RAO with higher doses of heparin (100 IU/kg) without an observed increase in bleeding complications.2 However, our analysis suggests that a fixed dose of 5000 IU offers comparable efficacy while simplifying procedural logistics and reducing the risk of dosing errors, particularly in high-volume centers or environments with limited resources. In our study, even at the extremes of dosage (< 50 IU/kg and > 80 IU/kg), the differences in RAO rates were not statistically significant.6
While meta-analyses have highlighted the benefits of higher doses of heparin, 5,11 including a significant reduction in RAO, our findings suggest that the baseline dose of 5000 IU offers a balanced approach, minimizing RAO without increasing bleeding risks. This is particularly relevant given the potential complications associated with high-intensity anticoagulation, such as hematomas or major bleeding. The lack of major bleeding events in our cohort underscores the safety of this approach.
The findings of our study differ slightly from the American Heart Association's guideline recommendations, which propose weight-based heparin dosing of 50 IU/kg for diagnostic procedures.3 This discrepancy might stem from differences in patient populations, procedural protocols, or baseline risk factors for RAO across studies. Additionally, the guidelines’ emphasis on weight-based dosing may reflect a theoretical benefit rather than consistent evidence from large-scale trials. Although weight-based adjustments might offer theoretical benefits, our results indicate that a fixed dose of 5000 IU is sufficient and simplifies procedural protocols without compromising outcomes.
Another point of comparison is the role of hemostasis. Studies advocating patent hemostasis techniques have shown lower RAO rates irrespective of heparin dosing, emphasizing the importance of hemostasis protocols.12 Our study uniformly employed short-duration pneumatic compression, which likely contributed to the low overall RAO rate observed.8
Limitations
This study is a subanalysis and may be influenced by the inherent limitations of the parent trial design. One limitation of our study is the absence of ultrasound guidance during radial artery access, which has been shown to reduce complications and improve success rates, as demonstrated by the RAUST trial.13 While this practice is becoming increasingly common, the original PATENS trial adhered to palpation-based techniques, which were standard at the time of its design. Future investigations should explore whether integrating ultrasound guidance could further mitigate RAO. Additionally, the study population was only composed of patients undergoing diagnostic procedures; results may not be generalizable to interventional settings.
Conclusions
Heparin doses adjusted by weight did not significantly influence the incidence of RAO in diagnostic TRA procedures. The findings highlight the potential for simplified, standardized anticoagulation protocols, which could enhance procedural efficiency in the catheterization laboratory. The standard dose of 5000 IU remains a safe and effective anticoagulation strategy for this patient population.
Affiliations and Disclosures
Roberto L. da Silva, MD, PhD1,2; Rodrigo M. Joaquim, MD1; Thaís R.W. da Silva, MD, MSc2; Felipe Borges Oliveira, MD1; Pedro B. de Andrade, MD, PhD3; José Ribamar Costa Jr, MD, PhD4
From the 1Department of Interventional Cardiology, Instituto de Cardiologia de Santa Catarina, São José, Brazil; 2Department of Cardiology, Hospital Universitário Prof Polydoro Ernani de São Thiago, Florianópolis, Brazil; 3Department of Interventional Cardiology, Santa Casa de Marília, Marília, Brazil; 4Department of Interventional Cardiology, Instituto Dante Pazzanese de Cardiologia, São Paulo, Brazil.
Disclosures: The authors report no financial relationships or conflicts of interest regarding the content herein.
Address for correspondence: Roberto Léo da Silva, MD, PhD, Department of Interventional Cardiology, Instituto de Cardiologia de Santa Catarina, Santa Catarina, Brazil. Email: roberto.leo@ufsc.br; X: @DrRobertoLeo
References
- Shroff AR, Fernandez C, Vidovich MI, et al. Contemporary transradial access practices: results of the second international survey. Catheter Cardiovasc Interv. 2019;93(7):1276-1287. doi:10.1002/ccd.27989
- Hahalis GN, Leopoulou M, Tsigkas G, et al. Multicenter randomized evaluation of high versus standard heparin dose on incident radial arterial occlusion after transradial coronary angiography: the SPIRIT OF ARTEMIS study. JACC Cardiovasc Interv. 2018;11(22):2241-2250. doi:10.1016/j.jcin.2018.08.009
- Mason PJ, Shah B, Tamis-Holland JE, et al; American Heart Association Interventional Cardiovascular Care Committee of the Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; Council on Peripheral Vascular Disease; and Council on Genomic and Precision Medicine. An update on radial artery access and best practices for transradial coronary angiography and intervention in acute coronary syndrome: a scientific statement from the American Heart Association. Circ Cardiovasc Interv. 2018;11(9):e000035. doi:10.1161/HCV.0000000000000035
- Rashid M, Kwok CS, Pancholy S, et al. Radial artery occlusion after transradial interventions: a systematic review and meta-analysis. J Am Heart Assoc. 2016;5(1):e002686. doi:10.1161/JAHA.115.002686
- Bossard M, Mehta SR, Welsh RC, Bainey KR. Utility of unfractionated heparin in transradial cardiac catheterization: a systematic review and meta-analysis. Can J Cardiol. 2017;33(10):1245-1253. doi:10.1016/j.cjca.2017.06.003
- Degirmencioglu A, Buturak A, Zencirci E, et al. Comparison of effects of low- versus high-dose heparin on access-site complications during transradial coronary angiography: a double-blind randomized study. Cardiology. 2015;131(3):142-148. doi:10.1159/000377621
- da Silva RL, de Andrade PB, Dangas G, et al. Randomized clinical trial on prevention of radial occlusion after transradial access using nitroglycerin: PATENS trial. JACC Cardiovasc Interv. 2022;15(10):1009-1018. doi:10.1016/j.jcin.2022.02.026
- da Silva RL, de Andrade PB, Abizaid AAC, et al. Comparison of minimum pressure and patent hemostasis on radial artery occlusion after transradial catheterization. J Invasive Cardiol. 2020;32(4):147-152. doi:10.25270/jic/19.00372
- Mehran R, Rao SV, Bhatt DL, et al. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation. 2011;123(23):2736-2747. doi:10.1161/CIRCULATIONAHA.110.009449
- Wagener JF, Rao SV. Radial artery occlusion after transradial approach to cardiac catheterization. Curr Atheroscler Rep. 2015;17(3):489. doi:10.1007/s11883-015-0489-6
- Tsigkas G, Papanikolaou A, Apostolos A, et al. Preventing and managing radial artery occlusion following transradial procedures: strategies and considerations. J Cardiovasc Dev Dis. 2023;10(7):283. doi:10.3390/jcdd10070283
- Bernat I, Aminian A, Pancholy S, et al; RAO International Group. Best practices for the prevention of radial artery occlusion after transradial diagnostic angiography and intervention: an international consensus paper. JACC Cardiovasc Interv. 2019;12(22):2235-2246. doi:10.1016/j.jcin.2019.07.043
- Seto AH, Roberts JS, Abu-Fadel MS, et al. Real-time ultrasound guidance facilitates transradial access: RAUST (Radial Artery access with Ultrasound Trial). JACC Cardiovasc Interv. 2015;8(2):283-291. doi:10.1016/j.jcin.2014.05.036





















