Transcatheter Tricuspid Valve Replacement for Rheumatic Tricuspid Valve Disease
© 2025 HMP Global. All Rights Reserved.
Any views and opinions expressed are those of the author(s) and/or participants and do not necessarily reflect the views, policy, or position of the Journal of Invasive Cardiology or HMP Global, their employees, and affiliates.
J INVASIVE CARDIOL 2025. doi:10.25270/jic/25.00249. Epub August 27, 2025.
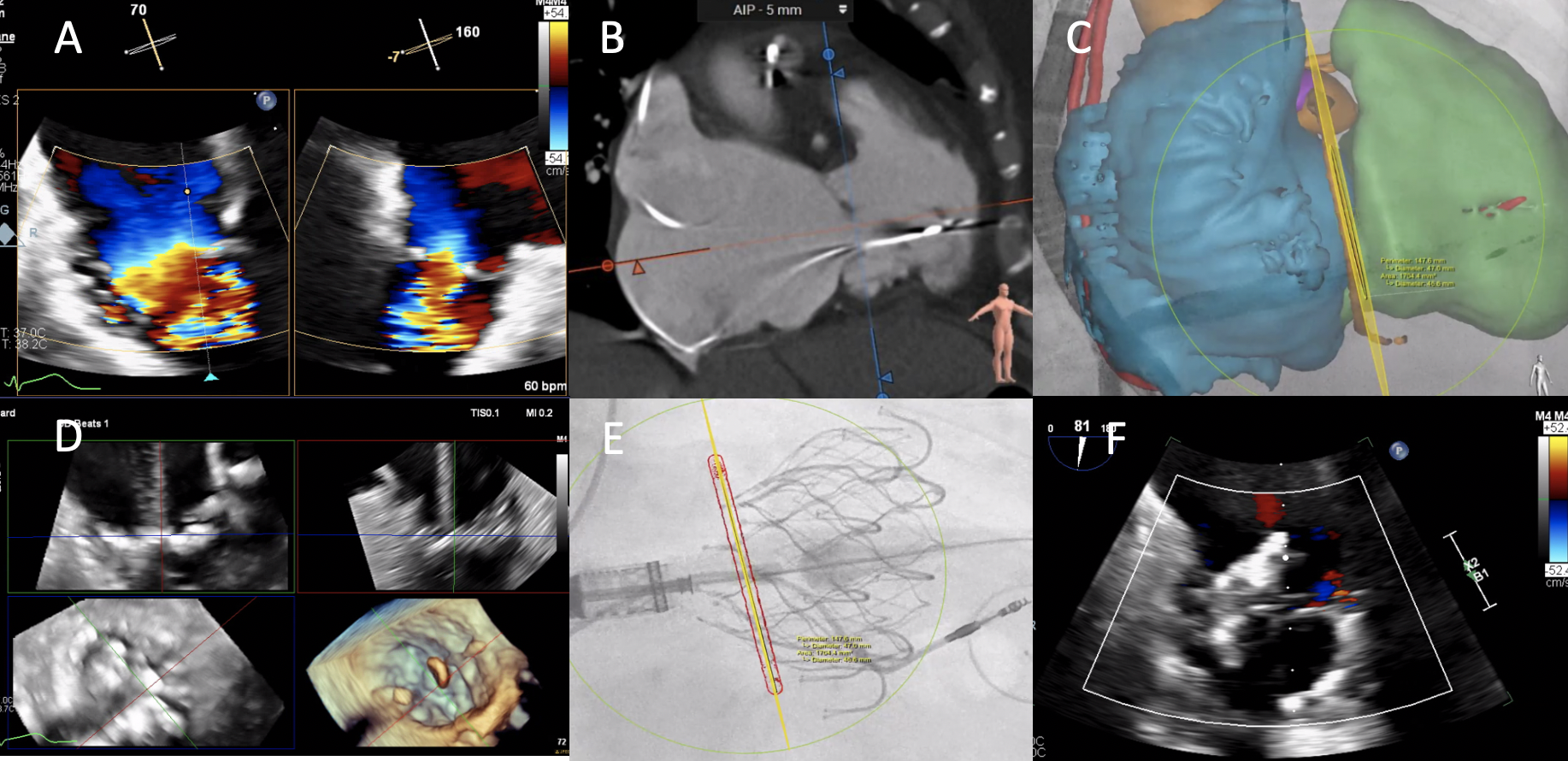
An 82-year-old woman with a history of rheumatic heart disease with mild mitral stenosis and severe tricuspid valve regurgitation/stenosis (Figure A) was referred to our center for transfemoral transcatheter tricuspid valve replacement (TTVR) with the EVOQUE device (Edwards Lifesciences) because of right-sided heart failure symptoms. Preoperative computed tomography (CT) demonstrated thickened tricuspid leaflets (Figure B) with annular sizing suitable for a 48-mm device.
The procedure was performed with transesophageal echocardiography (TEE) and CT-fluoroscopy fusion imaging guidance. The EVOQUE nosecone was advanced across the tricuspid annulus (Figure C) and anchor opening was performed with multiplanar reconstruction imaging guidance (Figure D). After the atrial side of the valve was released, there was slight tilting towards the septal commissure but excellent device function with trace residual intra-valvular leak (Figure E and F; Video). The patient had an uneventful hospital course and significant clinical improvement on follow-up at 6 months.
This case suggests that proper screening makes transcatheter replacement a feasible option for rheumatic tricuspid disease, expanding the potential reach of percutaneous therapies for this population.
Affiliations and Disclosures
Craig Basman, MD; Perry Wengrofsky, MD; Polydoros Kampaktsis, MD; Vandan Upadhyaya, MD; Rachel Spallone, MD; Sung-Han Yoon, MD; Ryan Kaple, MD
From Hackensack University Medical Center, Hackensack, New Jersey.
Disclosures: Dr Ryan Kaple is a consultant for Abbott and Edwards Lifesciences. The remaining authors report no financial relationships or conflicts of interest regarding the content herein.
Consent statement: The authors confirm that informed consent was obtained from the patient for the intervention described in the manuscript and for the publication thereof, including any and all images.
Address for correspondence: Craig Basman, MD, 30 Prospect Avenue 1 Link, Hackensack, NJ, 07601, USA. Email: craig.basman@hmhn.org; X: @craigbasman




















