Alterra and SAPIEN Frame Overexpansion for Repeated Valve-in-Valve Therapy
© 2025 HMP Global. All Rights Reserved.
Any views and opinions expressed are those of the author(s) and/or participants and do not necessarily reflect the views, policy, or position of the Journal of Invasive Cardiology or HMP Global, their employees, and affiliates.
The Alterra Adaptive Prestent (Edwards Lifesciences) received US Food and Drug Administration approval in December 2021 for use with the SAPIEN 3 transcatheter pulmonary valve (TPV) (Edwards Lifesciences) to treat severe pulmonary regurgitation in patients with a large, native, or surgically repaired right ventricular outflow tract. Given that all bioprosthetic valves eventually degenerate, pulmonary valve replacement strategies must balance durability, recovery, and future treatment options. Decisions should be tailored to patient anatomy, clinical condition, and long-term goals. For transcatheter approaches, counseling should address the feasibility of repeated interventions over a patient’s lifetime.
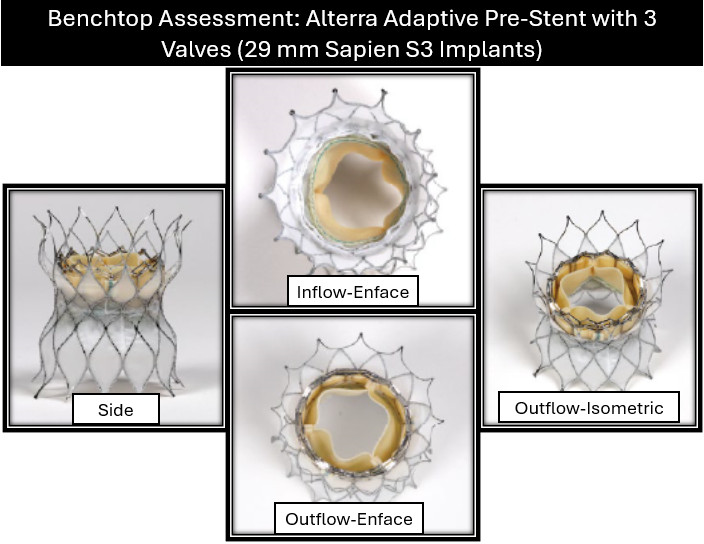
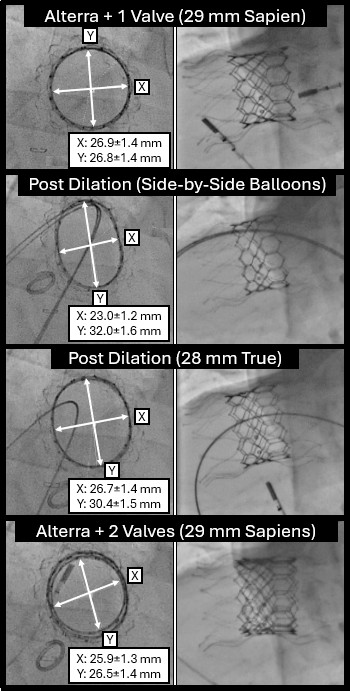
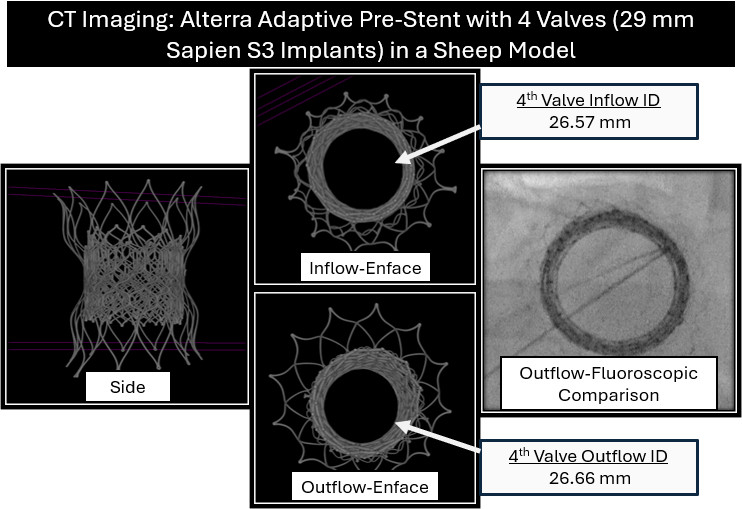
A key question with the Alterra Prestent and 29-mm SAPIEN 3 valve is what approach is optimal for repeated valve-in-valve (VIV) therapy. Current practice assumes each successive valve must be smaller, potentially limiting future options rather than considering the potential for frame overexpansion. To assess this, we evaluated over-expansion capabilities with serial VIV implants using benchtop (Figure 1) and in vivo sheep models. Over-expansion was achieved using a 2-balloon technique, inflating side-by-side 18-mm and 20-mm Atlas Gold balloons (BD) to 14 atmospheres, followed by circularization with a 28-mm True dilation balloon (BD) at 12 atmospheres followed by valve implantation. Repeat implantation of a 29-mm SAPIEN valve was then performed and the process was repeated. Post-implantation measurements confirmed that the desired specifications were still achieved after 4 total 29-mm SAPIEN 3 implants were placed in this manner (Figures 2 and 3).
This study demonstrates that the Alterra and SAPIEN 3 system can be overexpanded, enabling valve sizing based on achieved dilation rather than predetermined diameters. This flexibility may reduce redo sternotomies and minimize patient-prosthesis mismatch.
Affiliations and Disclosures
Jason H. Anderson, MD1,2; Gareth J. Morgan, MD3; Jenny E. Zablah, MD3; Allison K. Cabalka, MD1,2
From the 1Department of Pediatric and Adolescent Medicine/Division of Pediatric Cardiology, Mayo Clinic, Rochester, Minnesota; 2Department of Cardiovascular Medicine/Division of Structural Heart Diseases, Mayo Clinic, Rochester Minnesota; 3Department of Pediatrics/Division of Cardiology, Heart Institute, Children's Hospital Colorado, Aurora, Colorado.
Disclosures: Dr Anderson is on the cardiac advisory board for W.L. Gore & Associates and is a consultant for Edwards Lifesciences and Medtronic. Dr Morgan is a consultant for Edwards Lifesciences and Medtronic. Dr Zablah is a consultant for Edwards Lifesciences, Medtronic, B. Braun, Occlutech, and Philips. Dr Cabalka is a consultant for Edwards Lifesciences and B. Braun.
Funding: This study was funded by Edwards Lifesciences.
Address for correspondence: Jason H. Anderson, MD, Mayo Clinic, Rochester, MN 55905, USA. Email: Anderson.Jason@mayo.edu; X: @Dr_JHAnderson




















