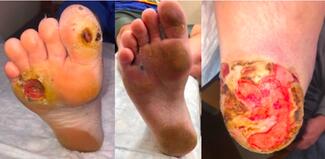
A Retrospective Comparison of Standalone and Combination Therapy Using Synthetic Extracellular Matrix Dressings and Amniotic Allografting in Lower Extremity Wounds
This retrospective evaluation takes a look at wound healing outcomes using synthetic extracellular matrix dressings, amniotic allografts, and combination therapy in lower extremity wounds of varying complexity.
Key Takeaways
- Comparable healing with monotherapy in similar wounds
Synthetic ECM dressings and amniotic allografts achieved equivalent wound volume reduction when used alone in lower extremity wounds with similar baseline severity. - Combination therapy supports more complex wounds
Despite treating wounds nearly 1.8 times more severe at baseline, combination therapy achieved meaningful wound volume reduction within six weeks, highlighting its role in challenging limb preservation cases. - Clinical flexibility without increased complications
All treatment approaches demonstrated strong safety profiles, with no infections, osteomyelitis, or amputations observed—supporting individualized modality selection based on wound depth, complexity, and care setting.
Introduction
Amniotic allografts and synthetic extracellular matrix (ECM) dressings are widely utilized advanced wound care modalities for the management of lower extremity wounds. In this study, the amniotic allograft used was PalinGen (Amnio Technology), and the synthetic ECM dressing was Anthem Wound Matrix, which is marketed within the Veterans Affairs health system and is also known commercially as Phoenix Wound Matrix (RenovoDerm) outside the VA system. Amniotic allografts provide a biologically active scaffold containing structural proteins, cytokines, and extracellular matrix components that support tissue repair and modulation of inflammation. Synthetic ECM dressings, particularly electrospun matrices, provide a three-dimensional scaffold that mimics native extracellular architecture and promotes cellular infiltration, wound stability, and tissue regeneration.1,2
Despite their individual effectiveness, concerns exist regarding the rapid degradation and clearance of amniotic allografts after application to the wound bed. Prior studies have demonstrated that electrospun synthetic matrices may improve cellular survival and create a more favorable microenvironment for tissue regeneration.3,4 Previous case series by Evensen and colleagues suggested that combination therapy using both modalities may offer synergistic benefits in complex lower extremity wounds.5,6
This study retrospectively compares outcomes of synthetic ECM dressings and amniotic allografts used as monotherapy versus combination therapy in lower extremity wounds treated within a Veterans Affairs healthcare system.
Methods
We conducted a retrospective review of anonymized patient data from the Southern Arizona Veterans Affairs Health Care System between July 2021 and October 2024. Patients were included if they were older than 18 years, had a lower extremity wound, and received synthetic ECM dressing monotherapy, amniotic allograft monotherapy, or combination therapy for a minimum of six consecutive weeks.
Twenty-one patients met inclusion criteria, with seven patients in each treatment arm. Wounds were managed across multiple clinics and providers using a standardized wound care protocol. Clinical decisions regarding offloading, secondary dressings, and antibiotic therapy were determined by the treating provider based on local protocol and clinical judgment.
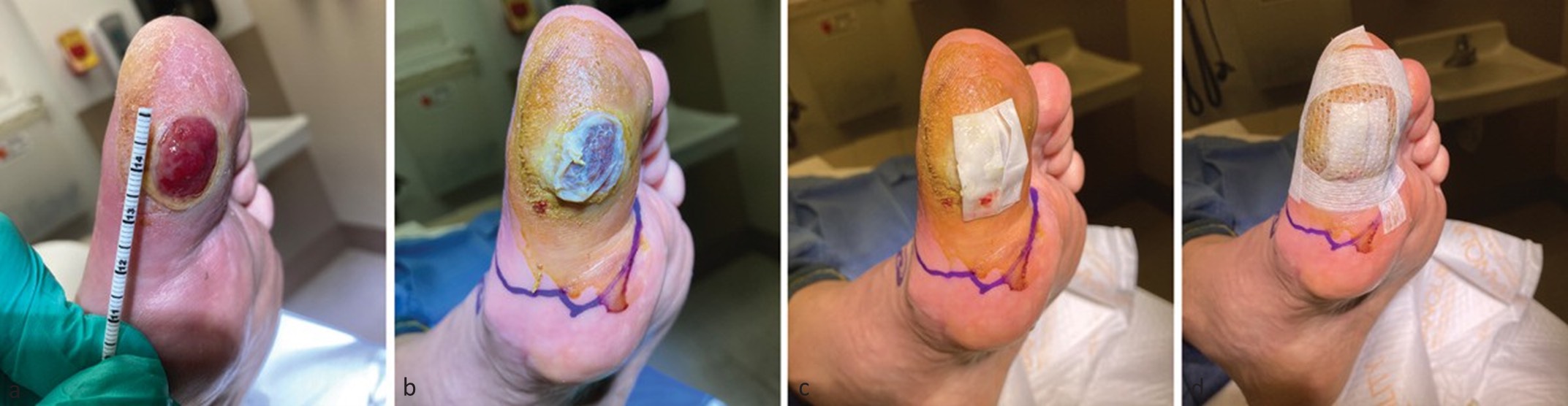
At each visit, wounds underwent sharp debridement, standardized cleansing, and documentation of wound characteristics. Synthetic ECM dressings were cut to size, hydrated in sterile saline, and applied directly to the wound bed. Amniotic allografts were applied as liquid formulations in wounds with exposed deep tissue, transitioning to membrane allografts once granulation tissue was present. In the combination therapy group, amniotic allografts were applied directly to the wound bed followed by placement of the synthetic ECM dressing over the graft.
Patients were evaluated weekly, and dressings remained intact between visits.
Data Analysis
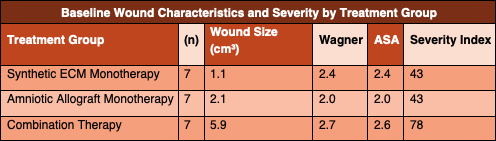
Initial wound volume was calculated using length, width, and depth measurements in cubic centimeters. Each wound was assigned a Wagner grade and an ASA physical status classification. A wound severity index was calculated by summing the rounded initial wound volume, Wagner grade, and ASA classification.
Wound volume was recorded at baseline and at each of six subsequent visits. Time to achieve 95 percent or greater wound volume reduction was calculated for each treatment arm. Wound trajectories were analyzed using graphical trend analysis.
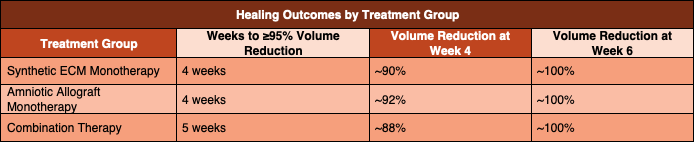
Results
All wounds achieved at least 95 percent reduction in wound volume within six weeks of initiating therapy. No patients developed new soft tissue infections, radiographic evidence of osteomyelitis, or required amputation during the treatment period.
Both the synthetic ECM monotherapy group and the amniotic allograft monotherapy group achieved the treatment goal of 95 percent wound volume reduction in an average of four weeks. These two groups had identical wound severity index scores, allowing for direct comparison of healing rates.
The combination therapy group achieved the treatment goal of 95 percent wound volume reduction in an average of five weeks. This group demonstrated a substantially higher baseline wound severity index, nearly 1.8 times greater than that of either monotherapy group, reflecting larger, deeper, and more complex wounds at presentation.
Discussion
Numerous studies support the use of amniotic allografts for lower extremity wound healing, with randomized controlled trials demonstrating improved healing rates compared with standard wound care.7,8 Electrospun synthetic ECM dressings have similarly demonstrated favorable outcomes, including accelerated wound closure and reduced complication rates.9,10
The results of this study demonstrate equivalent rates of wound volume reduction between synthetic ECM dressings and amniotic allografts when used as monotherapy in wounds of similar severity. This finding has important clinical implications, particularly when considering differences in cost, storage requirements, and ease of application between these modalities.
Although combination therapy demonstrated a slightly longer average time to wound volume reduction, this occurred in the context of significantly greater wound severity. When baseline wound complexity is taken into account, the findings support the role of combination therapy in the management of more severe or recalcitrant wounds, where enhanced scaffold stability and biologic support may be advantageous.
Limitations
This study is limited by its retrospective design, small sample size, and heterogeneity of wound etiologies and patient comorbidities. Treatment was delivered by multiple providers across different clinical settings, which may have introduced variability despite standardized protocols. The significantly higher wound severity index in the combination therapy group limits direct equivalence comparisons.
Clinical Implications
For clinicians managing lower extremity wounds, synthetic ECM dressings and amniotic allografts appear to provide comparable outcomes when used as monotherapy in appropriately selected wounds. Combination therapy may be particularly beneficial in wounds with greater depth, tissue loss, or delayed healing, especially in high-risk limb preservation populations.
Conclusion
Based on our findings, this retrospective comparison supports the use of synthetic ECM dressings and amniotic allografts as effective advanced wound care modalities. When used in combination, we feel that these therapies appear well suited for managing more severe lower extremity wounds and may offer meaningful advantages in complex limb preservation cases.
Disclosures. The authors of this article declare no conflict of interest. The companies involved had no role in the design of the study; in the collection, analyses, or interpretation of date; in the writing of the manuscript, or in the decision to publish the results.
Artificial intelligence (AI)–based tools were used in the preparation of this manuscript solely to assist with literature organization, language refinement, and reference formatting. All clinical interpretation, conceptual synthesis, and editorial oversight were performed by the authors. Human review and responsibility for accuracy, integrity, and originality were maintained throughout.
It is important to note that this case was performed at the Southern Arizona Veteran Affairs Health Care System, therefore cost and reimbursement were not factors in determining treatment. Anthem Wound Matrix (RenovoDerm) is branded specifically for the Department of Veteran Affairs. In private and commercial facilities, it is branded as the Phoenix Wound Matrix. Amniotic allografts, and synthetic extracellular matrices are all reimbursable through Medicare and private insurance and have their own designated HCPCS codes. The cost-to-benefit ratio would need to be assessed on an individual provider and patient basis. All pricing of the wound care products used in this case are available to the public through the Department of Veteran Affairs Federal Supply Schedule.
Acknowledgements. This material is based upon work supported by the Department of Veterans Affairs, Veterans Health Administration, and Office of Research and Development. The authors gratefully acknowledge the Southern Arizona VA Health Care System which provided facilities and materials for this research.
This study met Institutional Review Board exemption under 38CFR16.104 and qualified for exemption under category 4(iiii). The waiver of HIPAA authorization was approved as outlined and described in VA Central IRB Form 103 signed by the designated VA Central IRB representative. All criteria for granting the waiver as specified in 45 CFR 164.512 was met.
Dr. Evensen is the Chief Podiatry Resident in the Department of Veteran Affairs at Southern Arizona Veteran Affairs Healthcare System in Tucson, Arizona.
Dr. Walters is a Diplomate of the American Board of Foot and Ankle Surgery and a podiatrist in the Department of Veteran Affairs at Southern Arizona Veteran Affairs Healthcare System in Tucson, Arizona.
Dr. Johnston is a diplomate of the Board of Wound Management and a in the Department of Veteran Affairs at Southern Arizona Veteran Affairs Healthcare System in Tucson, Arizona.
Nurse Sepulveda is a wound care nurse practitioner in the Department of Veteran Affairs at Southern Arizona Veteran Affairs Healthcare System in Tucson, Arizona.
Dr. Samoy is a Diplomate of the American Board of Podiatric Medicine and a podiatrist in the Department of Veteran Affairs at Southern Arizona Veteran Affairs Healthcare System in Tucson, Arizona.
Dr. Dancho is a Fellow of the American College of Foot and Ankle Surgeons and the American College of Podiatric Medicine. He is a podiatrist in the Department of Veteran Affairs at Southern Arizona Veteran Affairs Healthcare System in Tucson, Arizona.
Dr. Jolley is a Diplomate of the American Board of Podiatric Medicine and a podiatrist in the Department of Veteran Affairs at Southern Arizona Veteran Affairs Healthcare System in Tucson, Arizona.
Ms. Mills is affiliated with the Biomedical Research and Education Foundation of Southern Arizona in the Department of Veteran Affairs at Southern Arizona Veteran Affairs Healthcare System in Tucson, Arizona.
References
1. Schmiedova I, Ozanova Z, Stastna E, et al. Case report: freeze-dried human amniotic membrane allograft for the treatment of chronic wounds: results of a multicentre observational study. Front Bioeng Biotechnol. 2021;9:649446.
2. Miguel SP, Figueira DR, Simoes D, et al Electrospun polymeric nanofibres as wound dressings: a review. Colloids Surf B Biointerfaces. 2018;169:60–71.
3. Lambert CJ Jr, Aviles F Jr, Eckert KA, et al. Efficacy of a 3D electrospun synthetic polymer matrix on hard-to-heal wounds. Surg Technol Int. 2022;43:31–38.
4. Lee CH, Shin HJ, Cho IH, et al. Nanofiber alignment and direction of mechanical strain affect the ECM production of human ACL fibroblast. Biomaterials. 2005;26:1261–1270.
5. Evensen A, Walters J, Dancho J, Samoy V, Jolley D. Impact of synthetic extracellular matrices in combination therapy with amniotic allografting in the treatment of diabetic foot wounds: a case series. Surg Technol Int. 2024;44:53–60.
6. Evensen A, Curbo L, Batra S. Treatment of an exposed Achilles tendon within a refractory mixed arterial venous leg ulcer with the novel use of pericardium allograft in combination with amniotic allografting, synthetic extracellular matrix, and acellular dermis allografting: a case report. Surg Technol Int. 2024;44:66–70.
7. Lavery LA, Fulmer J, Shebetka KA, et al. Grafix Diabetic Foot Ulcer Study Group. The efficacy and safety of Grafix for the treatment of chronic diabetic foot ulcers: results of a multi-centre, controlled, randomised, blinded, clinical trial. Int Wound J. 2014;11:554–560.
8. Zelen CM, Serena TE, Denoziere G, Fetterolf DE. A prospective randomised comparative parallel study of amniotic membrane wound graft in the management of diabetic foot ulcers. Int Wound J. 2013;10:502–507.
9. Horn CP, Fierro AL, Lantis JC. The shift to synthetics: a review of novel synthetic matrices for wound closure. Surg Technol Int. 2023;42.
10. Khalid H, Malik A, Kirchens J, Choi G. A prospective, blinded, randomized controlled clinical trial evaluating the effect of the synthetic electrospun fiber matrix in the treatment of chronic diabetic foot ulcers. Foot Ankle Surg Tech Rep Cases. 2024;4:100362.
11. Hocking AM, Gibran NS. Mesenchymal stem cells: paracrine signaling and differentiation during cutaneous wound repair. Exp Cell Res. 2010;316:2213–2219.
12. Kim HN, Hong Y, Kim MS, et al. Effect of orientation and density of nanotopography in dermal wound healing. Biomaterials. 2012;33:8782–8792.
© 2026 HMP Global. All Rights Reserved.
Any views and opinions expressed are those of the author(s) and/or participants and do not necessarily reflect the views, policy, or position of Podiatry Today or HMP Global, their employees, and affiliates.