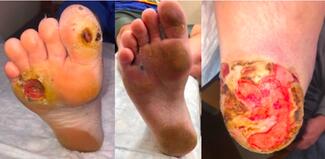
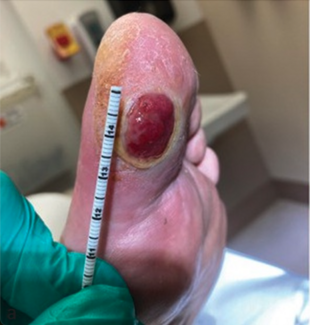
Regenerative Medicine in Podiatry: From Buzzword to Practice-Changing Reality
Regenerative medicine is rapidly reshaping how podiatrists approach musculoskeletal injury, chronic pain, wound care, and patient expectations. Foot and ankle specialists are uniquely positioned to lead the responsible integration of such technologies. This author offers her experience with a practical roadmap for exploring the regenerative frontier.
Key Takeaways
- Podiatrists Are Strategically Positioned to Lead Regenerative Care
With deep expertise in biomechanics, soft tissue pathology, wound management, and limb preservation, podiatrists are natural leaders in applying regenerative and restorative therapies for lower extremity conditions. - Patient Demand Is Shifting from Pain Relief to True Recovery
Today’s patients actively seek treatments that promote healing, function, and long-term vitality, creating both a clinical opportunity and a practice growth pathway with thoughtful adoption. - Successful Integration Requires Education, Strategy, and Trusted Partnerships
Regenerative medicine remains a rapidly evolving and inconsistently regulated space; podiatrists must prioritize staff education, evidence-based protocols, and collaboration with reputable, well-established industry partners to uphold safety, outcomes, and professional integrity.
This article discusses emerging modalities. Regulatory status and evidence quality vary by product and indication; clinicians should consult FDA labeling, state regulations, and current literature before offering any therapy.
In the past 18 months, our practice has seen the “new” category of healthcare that is regenerative medicine explode onto the podiatric landscape. Just in the past 3 months, Lexington Podiatry has onboarded 4 new regenerative treatment options, and our patient feedback is very positive.
Not only do our patients appreciate these exciting and advanced new offerings, but these options inspire our providers with a newfound vigor in their patient interactions and treatment plans. After nearly 20 years of practice, I find it thrilling to chat about these newly available treatment options. The patient feedback and current Google trends in patient care indicate a growing demand for more than just pain relief.1 Patients are actively searching for recovery, wellness, and overall vibrancy.
Given podiatrists’ primary expertise in lower extremity biomechanics, soft tissue pathology, and the various foot complications we encounter; it is only logical that we take the initiative in spearheading the integration of regenerative medicine within our practice for a variety of foot and ankle disorders.
As a sports and biomechanical specialist, I have observed a massive surge in patient interest in alternatives to traditional treatment options. Personally, I treat a lot of patients who are on their second or third, if not fourth opinion looking for answers that align with their lifestyle. They’re no longer looking to simply mask pain. They want to repair and heal to yield the best outcome during recovery. They’re not interested in a quick fix but are interested in a long-term health investment.
This topic reminds me of one of my favorite books, Don’t Move My Cheese.2 This short read applies to all of us (including your staff). Simply the thought of incorporating these nuanced medical treatments can feel downright “scary” for you and your team. As we know, as a whole, our employees dislike change. But change is inevitable, especially in private practice. Thus, moving forward and having a solid execution strategy to educate your staff prior to recommending these treatment options is imperative.
Speaking of education, I find that the resources currently available are lacking. There’s so much information available, where do we start? What’s accurate? Not only is it time-consuming to sift through all this new medical jargon, but nothing seems to be consistent and straightforward. My advice, when reviewing all the case studies and literature, is to lean on the experts in this field. This isn’t anything we learned in podiatry school, so we must tread lightly when onboarding these new modalities and partner with seasoned experts. Also, it is wise to be very cognizant of the regulatory approval status of any modality you investigate, and to examine the associated risks versus benefits and your comfort level with that status.
If you’re still reading this, you are likely interested in exploring these options further. Below are some of the top options we utilize in our practice.
A Closer Look at Regenerative Options for the Lower Extremity
Systemic Peptides. What exactly is a peptide? Peptides are chemical bonds that link short chains of amino acids together. They are naturally occurring hormones, neurotransmitters and enzymes.3 Think of peptides as the “text messengers” of the body. They have been studied in multiple physiologic roles, from growth hormone signaling and collagen stimulation to tissue healing, muscle regeneration, and anti-aging support. Peptides work to improve remodeling of tendons, increase microcirculation, control inflammatory responses, and enhance recovery after an injury, surgical procedure, or overuse; all of which are within our clinical environment. 4-7 I’ve even learned that some individuals inject peptides to get a tan—although that’s not a recommendation for a podiatric indication.8
Most patients don’t realize that peptides have been used since the early nineteenth century when amino acids, the building blocks of peptides and proteins, were isolated. They were artificially synthesized in the 1960s.9 Most prescribers don’t know that insulin is a peptide, thus, these compounds are nothing new to medicine.10 As advancements in molecular biology and biochemistry progressed, researchers mapped sequences to unveil critical functional roles. Recently, with the assistance of glucagon-like peptide-1 (GLP-1) drugs, the field of these therapies is advancing, and I’m seeing a more abundant, widespread introduction of peptides in healthcare.
However, one should note that most systemic peptides are not currently FDA-approved for human use. There are specific, synthetic peptides that have FDA approval for specific endocrine disorders, but outside of those use cases, we have yet to see systemic peptides approved from a regulatory perspective.11 Options to look out for more information and research as they emerge for musculoskeletal or neuroprotective benefits include combinations of body protection compound (BPC)-157 and thymosin beta (TB)-500, which currently fall under “research use only” designations.12 Nicotinamide adenine dinucleotide (NAD)+ is another example to familiarize yourself with.13
Locally-Injected Peptides. In-office, local injectable peptide therapy involves delivering amino acid sequences directly to the injured area with a goal of promoting tissue repair and modulating inflammatory responses, potentially aiding recovery for various musculoskeletal disorders. Similar to systemically administered peptides, locally injected peptides (eg, for joint pain or tissue healing) lack FDA approval for human therapeutic use and are considered unapproved drugs when marketed or administered clinically. Many such peptides have been identified by the FDA as presenting significant safety risks and were excluded from authorized compounding between 2023 and 2025, regardless of route of administration.11
One must also keep in mind safety and complication considerations, as one should with any medication. These include but are not limited to allergic reactions; injection site issues; hormonal changes; metabolic impacts; drug interactions. There are also contraindications to consider, including, but limited to: pregnancy and breastfeeding; autoimmune conditions; kidney or liver issues; allergic history; and those with cancer or tumor conditions.14
Our experience has been positive with systemic and local peptides, including coordinating with other treatment modalities. Our hope is that more robust clinical trial data and larger-scale human studies will be forthcoming.
Infrared Light Therapy—Photobiomodulation. In our experience, this is yet another stage of wellness optimization. Through photobiomodulation, red light therapy amplifies the production of adenosine triophosphate (ATP) at the cellular level.15,16 Mitochondrial chromophores (mainly cytochrome c oxidase) absorb light, which then can increase ATP and decrease oxidative stress and inflammatory mediators.17 This is a regionally-acting, nondrug approach that aims to reduce pain and assist tissue healing in patients with conditions like chronic plantar fasciitis or tendinopathies.18
Many low-level light therapy (LLLT) or photobiomodulation (PBM) devices have received FDA 510(k) clearance for specific clinical indications such as the temporary relief of minor pain and inflammation associated with musculoskeletal conditions.19 In addition, in November 2024, the U.S. FDA authorized the Valeda Light Delivery System—a photobiomodulation device—for treating dry age-related macular degeneration (AMD) based on clinical evidence of improved visual acuity.20 This represents one of the first FDA-authorized therapeutic PBM devices for a defined clinical indication.
A standard infrared capsule is an all-encompassing, fully immersive, full-body light therapy system designed with the aim of stimulating self-healing, recovery, and cellular renewal at different wavelengths:17
- Infrared/Near-infrared (~850 nm): Commonly used in PBM protocols aimed at supporting recovery and comfort, with proposed mechanisms involving mitochondrial signaling and inflammatory modulation. Clinical effects depend on dosing, frequency, and indication.
- Deep near-infrared (~1060 nm): Often used for thermal stimulation and has been studied in select settings related to body contouring/temperature-based effects; claims related to metabolism or visceral fat should be interpreted cautiously and are device- and protocol-dependent.
- Red light (~630 nm): This wavelength has been studied evaluating outcomes such as appearance and collagen-related signaling; results vary.
- Yellow/amber (~590 nm): Used in some light-based protocols aimed at skin appearance (eg, redness); evidence is less consistent than for red/blue wavelengths.
- Blue light (~450 nm): Known for surface-level antimicrobial effects and commonly used for skin clarity; it is not generally used for deep tissue targets. There is some discussion, however, of nervous system impacts.21
Incorporating red light therapy as an add-on, fee-for-service option for neuropathy to chronic tendonitis (amongst many others) has support in the literature.22,23 Therapy includes two to three sessions weekly, for three to four weeks, in combination with stretching, load management, changes to footwear, orthotics, and shockwave therapy, etc, if appropriate.
Contraindications and precautions vary by device labeling and clinical context; but there are commonly cited precautions. Unlike pharmaceuticals, photobiomodulation is generally well tolerated, but mild and transient side effects have been documented, including skin reactions; short-term pain or numbness; eye sensitivity or injury (without protective eyewear); photosensitivity effects; rare thermal effects.24 Systematic reviews generally find no consistent pattern of serious adverse effects directly attributable to PBM when used within established protocols, though documentation of rare outcomes remains limited.24
Absolute contraindications include but are not limited to direct eye exposure and unshielded treatment over confirmed malignancies. Relative contraindications include but are not limited to pregnancy, photosensitive conditions or medications, seizure disorders, open wounds, active infection, severe acute inflammation, electronic implants, or pacemakers.25
Light Therapy Mat. A light therapy mat can energize, enhance recuperation, and optimize the functionality of one’s cells. Because of the ubiquity of energizing and stimulating light wavelengths, one can achieve and maintain deep cellular restoration, and muscle recuperation, as well as holistic wellness at any location.26 We have found that this technology can seamlessly incorporate into the workflows of a podiatry practice and can serve as a solo service or contribute to a more comprehensive regenerative medicine service.
Full-body clinical-grade light therapy mats that use red and near-infrared light are part of the broader class of photobiomodulation/LLLT devices, many of which have received FDA 510(k) clearance as Class II medical devices when supported by evidence for specific indications such as temporary relief of pain, inflammation, and musculoskeletal symptoms.27 Reported adverse effects are generally mild and transient, and are generally the same as that previously discussed for photobiomodulation.24-25
Commercial-Grade Infrared Sauna. This is a unique apparatus that can stand up to high traffic, commercial use.28,29 Far- and near-infrared sauna therapy has evidence that supports improvement of blood flow and endothelial performance.30 Regular use of such saunas post-exercise has also been associated with enhanced neuromuscular recovery and reduced subjective muscle soreness, and systematic reviews of infrared radiation for musculoskeletal conditions support its role in pain relief and functional improvement.31,32 Contraindications include, but are not limited to, cardiovascular issues, pregnancy, open wounds, cancers, or certain skin conditions. Complications can include dehydration or heat exhaustion with overexposure, burns, or eye damage without protective eyewear.33,34
Infrared saunas marketed for general wellness are not FDA-approved to treat disease; therapeutic disease claims would require appropriate FDA clearance/approval. The FDA has issued warning letters indicating that infrared saunas marketed with claims that they treat or cure diseases are being sold without required marketing clearance or approval, reinforcing that these devices are not FDA-approved medical treatments.35
Stem Cell Therapy. Diabetic foot ulcers (DFUs) remain one of the highest stakes problems in medicine, with major implications for amputation risk, hospitalizations, and cost. We are often the primary clinicians coordinating offloading, debridement, infection control, and biomechanical management. We are therefore perfectly positioned to lead the integration of advanced biologic therapies as they become approved. I feel that stem‑cell–based therapies are rapidly advancing diabetic wound care and tissue regeneration. However, one must note that these therapies specifically for DFU are not FDA-approved at this stage and are instead considered investigational.36 In the US, FDA-approved stem cell products are primarily hematopoietic; most other stem cell uses remain investigational outside clinical trials.
There are some investigations that suggest cell-based therapies could contribute to diabetic wound care however, by supporting tissue repair by secreting growth factors and cytokines.37 They could also facilitate angiogenesis38 and reduce chronic inflammation that impedes healing.39
Exosomes. Exosome-based treatment approaches are also emerging. Unlike peptides (signaling molecules for amino acids), exosomes are vesicles that mediate communication between cells by carrying a complex cargo of proteins and RNA.40,41
Two groups of researchers reviewed evidence that mesenchymal stem cell (MSC)-derived exosomes enhance fibroblast proliferation, angiogenesis, and collagen remodeling in anything from arthritic joints to tendonitis to chronic wounds.40,41 Although many studies remain preclinical or early-phase, in my observation, the direction is clear: future progressive medical practices will integrate cellular or cell-free biologics, and we as podiatrists are poised to be at the center of this shift.
At present, exosome therapy is not FDA-approved for medical, therapeutic, or cosmetic use and they are strictly experimental and regulated as drugs/biologics, requiring phase I-III trials. They may not be marketed outside of clinical trials at this time.42 I am optimistic about what the future will bring for this modality.
Cryotherapy. Another concept undergoing rapid development in medicine is the advancement of cryotherapy. We have used various types of cryotherapy practices and techniques, including ice therapy, cold pack therapy, cold compression devices, and cryo-air units. Cooling injured tissues leads to a decrease in nerve conduction speed and metabolic tissue demands in addition to making tissue inflammation mediators and their resultant swelling temporarily go away.43-45 This phenomenon clinically intersects various injuries and stages in our practice, eg acute-ankle sprains, postoperative recovery and follow-ups post bunion or hindfoot surgeries, or procedures like those pertaining to athletics. Be on the lookout for new devices in this category. One should note that whole body cryotherapy is not FDA-cleared or approved for the treatment of any specific medical conditions.46
Class IV MLS Laser Therapy. This FDA-cleared, non-invasive treatment modality employs specific wavelengths using a Multiwave Locked System (MLS). These wavelengths are designed to reduce pain and inflammation, accelerate healing and improve mobility. In our experience, it’s particularly beneficial for those suffering with acute and chronic Achilles tendonitis, sprains, or even fractures by promoting angiogenesis.47,48 We find in our practice that two to three treatments a week for two to three weeks can significantly reduce patients’ pain. MLS Class IV laser devices have received FDA clearance for therapeutic applications such as reduction of pain and inflammation and promotion of soft tissue repair through synchronized dual-wavelength laser emissions that enhance cellular and tissue responses.49
Shockwave Therapy. By utilizing high-frequency acoustic waves, this technology stimulates cellular repair, increases blood flow, and promotes tissue regeneration.50 It is particularly effective in treating soft tissue injuries, such as chronic Achilles tendonitis. Therapy sessions range from three to five sessions, typically one week apart and take roughly 20 minutes each. The device delivers focused acoustic waves to the affected area. In our experience, patients find this method particularly effective as they can guide you to the “hot spot.” Once you reach the injured area, we see that the pain often starts to dissipate and the goal of healing can begin.
Some shockwave therapy devices typically achieve clearance through the FDA 510(k) process by demonstrating substantial equivalence to legally marketed predicate devices for defined therapeutic use claims; novel or expanded indications may require additional regulatory review.51
Fat Pad Restoration. By utilizing injectable cryopreserved human adipose as a tissue allograft we can potentially help a wide range of patients. From metatarsalgia to inferior calcaneal fat pad atrophy, and even painful hammertoes, in our practice, we’re able to work to pad and cushion bony prominences. This can offer immediate support by stimulating the body’s own tissue.52,53 As a result, patients may experience long-lasting relief from pressure, pain and calluses by restoring or improving shock absorption.
Clinical use of adipose tissue allografts for fat pad restoration may be considered off-label or investigational, depending on product characteristics, degree of processing, and intended use under FDA regulatory frameworks.54
Conclusion
In today’s rapidly evolving healthcare landscape, especially for those in private practice, success hinges on our ability to swiftly anticipate and adapt to change—evolve or die. By diligently scrutinizing the literature, closely monitoring FDA decisions, and thoughtfully weighing the risks versus benefits of each treatment option, we can navigate this new terrain with confidence. As we embrace the unknown, let us remain steadfast in our commitment to our oath: to 'do no harm' as we guide our patients toward the best possible outcomes.
From my perspective, integrating regenerative medicine modalities is the wave of the future. Tailored protocols for common diagnoses can allow practitioners that choose these options work to enhance healing, improve patient outcomes, and establish themselves as leaders in advanced regenerative treatments.
However, I’m a realist. It’s not as easy as it sounds and it can be downright frightening if you don’t know where to start. Additionally, at the current moment it is the wild-wild west for regenerative medicine, due to poor regulation oversight. Therefore, it is critical that you find reputable companies that have been in this industry for decades. Collaborate with vetted and trusted companies that can support you with clinical studies, proven purity, and robust resources to educate your patients. Keep an eye on regulatory decisions, robust peer-reviewed literature, and real-world evidence. Learn more about solo and combination therapies, as the evidence evolves. Biostacking, specifically biostacking hyperthermic pods, is one such modality to look out for.
Given the ever-evolving landscape in podiatry, by “moving your cheese” you can optimize patient care and your bottom line. We must not fear change but embrace it as a path to doing something better. Let’s go of the past and move forward. It's time to saddle up, for tonight we ride!
Dr. Freels is the Founder and CEO of Lexington Podiatry and Modern Podiatrist. She discloses that she is a Faculty Member of MedBridge Global.
References
1. Google Trends. Regenerative medicine search trends, United States, past 5 years. https://trends.google.com/explore?q=regenerative%20medicine&date=today%205-y&geo=US. Accessed January 28, 2026.
2. Johnson S. Who Moved My Cheese? New York, NY: G.P. Putnam’s Sons; 1998.
3. StatPearls. Peptide hormones. National Center for Biotechnology Information. https://www.ncbi.nlm.nih.gov/books/NBK562260/. Accessed January 28, 2026.
4. Choi E, Kim M, Lee DH, et al. Functional Benefits of Low-Molecular-Weight Collagen Peptide in Achilles Tendon and Medial Collateral Ligament Injuries and Anterior Cruciate Ligament Transection-Induced Osteoarthritis. J Microbiol Biotechnol. 2025 Sep 22;35:e2506035. doi: 10.4014/jmb.2506.06035. PMID: 41016815; PMCID: PMC12535853.
5. Sokolova, I.B., Sergeev, I.V., Ryzhak, G.A. et al. The effect of vascular peptide bioregulator on microcirculation in the brain cortex of old rats. Adv Gerontol. 2016;6:328–332. https://doi.org/10.1134/S2079057016040135
6. La Manna S, Di Natale C, Florio D, Marasco D. Peptides as Therapeutic Agents for Inflammatory-Related Diseases. Int J Mol Sci. 2018;19(9):2714. doi: 10.3390/ijms19092714. PMID: 30208640; PMCID: PMC6163503.
7. Jeyanthi P, Gulothungan G, Emran TB. Peptide drugs and their use in surgical treatments. Int J Surg Open 2024;62(5):668-669. DOI: 10.1097/IO9.0000000000000152
8. Fosgerau K, Hoffmann T. Peptide therapeutics: current status and future directions. Drug Discov Today. 2015;20(1):122-128.
9. Apostolopoulos V, Bojarska J, Chai TT, et al. A Global Review on Short Peptides: Frontiers and Perspectives. Molecules. 2021;26(2):430. doi: 10.3390/molecules26020430. PMID: 33467522; PMCID: PMC7830668.
10. StatPearls. Peptides at the molecular level. National Center for Biotechnology Information. https://www.ncbi.nlm.nih.gov/books/NBK525983/. Accessed January 28, 2026.
11. U.S. Food and Drug Administration. Certain bulk drug substances for use in compounding that may present significant safety risks. FDA; 2025. https://www.fda.gov/drugs/human-drug-compounding/certain-bulk-drug-substances-use-compounding-may-present-significant-safety-risks. Accessed January 26, 2026.
12. Vasireddi N, Hahamyan H, Salata MJ, et al. Emerging Use of BPC-157 in Orthopaedic Sports Medicine: A Systematic Review. HSS J. 2025 Jul 31:15563316251355551. doi: 10.1177/15563316251355551. Epub ahead of print. PMID: 40756949; PMCID: PMC12313605.
13. American Pharmacists Association. NAD supplements: regulatory and safety considerations. https://www.pharmacist.com/Blogs/CEO-Blog/Article/nad-supplements. Accessed January 28, 2026.
14. International Review Board of Laser Safety. Peptide safety: understanding risks, side effects, and responsible use. IRBLS; 2025. Accessed January 26, 2026.
15. Dompe C, Moncrieff L, Matys J, et al. Photobiomodulation-Underlying Mechanism and Clinical Applications. J Clin Med. 2020;9(6):1724. doi: 10.3390/jcm9061724.
16. Glass GE, et al. Photobiomodulation: the clinical applications of low-level light therapy. Aesthet Surg J. 2021;41(6):723-738. doi:10.1093/asj/sjab025.
17. Hamblin MR. Mechanisms and applications of the anti-inflammatory effects of photobiomodulation. AIMS Biophys. 2017;4(3):337-361. doi:10.3934/biophy.2017.3.337.
18. Chaves RA, et al. Photobiomodulation therapy in the treatment of pain: a systematic review. Photomed Laser Surg. 2018;36(11):561-577.
19. Blue Cross Blue Shield of Michigan. Medical policy: low level laser therapy and high-power laser systems. Policy No. 76911.
20. Sadda SR. Photobiomodulation for Age-Related Macular Degeneration. JAMA Ophthalmol. 2025 Mar 1;143(3):195-196. doi: 10.1001/jamaophthalmol.2025.0077. PMID: 39854014.
21. Liu Y, et al. Photobiomodulation for the treatment of neurological disorders: a review. J Photochem Photobiol B. 2018;185:198-206. doi:10.1016/j.jphotobiol.2018.07.014
22. Cinar E, Saxena S, Uygur F. Low-level laser therapy in the management of plantar fasciitis. In: Lasers in Medical Science. Springer Medicine. https://www.springermedicine.com/low-level-laser-therapy-in-the-management-of-plantar-fasciitis-a/21398294. Accessed January 28, 2026.
23. Wang Y, et al. Effectiveness of photobiomodulation therapy: a systematic review. BMJ Open. 2022;12:e059479.
24. Esteves-Pereira TC, Brandão TB, Bensadoun R-J, Fontes EK, Paglioni MdP, Santos-Silva AR. Safety and adverse effects in photobiomodulation therapy. In: Photobiomodulation Therapy in Oral Medicine. Springer Nature; 2025:27-29. doi:10.1007/978-3-031-85048-6_5.
25. Harrington P. Review of photobiomodulation contraindications. Acupuncture Today. 2025. https://acupuncturetoday.com/article/34288-review-of-pbm-contraindications. Accessed January 28, 2026..
26. Kazemi M, et al. Light therapy and its effects on cellular function. Int J Sports Med. 2018;39(4):267-275. doi:10.1055/a-0654-4291
27. U.S. Food and Drug Administration. Photobiomodulation devices—510(k) guidance. https://www.fda.gov/media/164417/download. Accessed January 28, 2026.
28. Ahokas EK, Ihalainen JK, Hanstock HG, Savolainen E, Kyröläinen H. A post-exercise infrared sauna session improves recovery of neuromuscular performance and muscle soreness after resistance exercise training. Biol Sport. 2023 Jul;40(3):681-689. doi: 10.5114/biolsport.2023.119289. Epub 2022 Sep 15. PMID: 37398966; PMCID: PMC10286597.
29. Sugie M, Harada K, Takahashi T, et al. Effectiveness of a far-infrared low-temperature sauna program on geriatric syndrome and frailty in community-dwelling older people. Geriatr Gerontol Int. 2020 Oct;20(10):892-898. doi: 10.1111/ggi.14003. Epub 2020 Aug 9. PMID: 32776407; PMCID: PMC7590093.
30. Tei C, et al. Waon therapy for cardiovascular disease and peripheral arterial disease. Circ J. 2010;74:617-621.
31. Ahokas EK, et al. Effects of a post-exercise infrared sauna session on neuromuscular performance and muscle soreness. Biol Sport. 2023;40(3):681-689.
32. Tsagkaris C, et al. Infrared radiation in the management of musculoskeletal conditions and chronic pain. Eur J Investig Health Psychol Educ. 2022;12(3):334-343.
33. Hussain J, Cohen M. Clinical effects of regular dry sauna bathing: a systematic review. Evid Based Complement Alternat Med. 2018;2018:1857413. doi:10.1155/2018/1857413
34. Kukkonen-Harjula K, Kauppinen K. Health effects and risks of sauna bathing. Int J Circumpolar Health. 2006;65(3):195-205.
35. U.S. Food and Drug Administration. Warning letter: Living Foods LLC. July 5, 2022. https://www.fda.gov/inspections-compliance-enforcement-and-criminal-investigations/warning-letters/living-foods-llc-622648-07052022. Accessed January 28, 2026.
36. U.S. Food and Drug Administration. Important patient and consumer information about regenerative medicine therapies. FDA; 2024.
37. Liu Y, Wang J. Mesenchymal stem cells for the treatment of diabetic foot ulcers: a systematic review and meta-analysis. Cell Transplant. 2016;25(6):1027-1036. doi:10.3727/096368916X693434
38. Zhang Y, et al. Neovascularization induced by mesenchymal stem cells for diabetic wounds. Stem Cells Int. 2018;2018:4567430. doi:10.1155/2018/4567430
39. Follin B, et al. The role of mesenchymal stem cells in the resolution of inflammation. Stem Cells Transl Med. 2019;8(5):552-558. doi:10.1002/sctm.18-0358
40. Chen B, Chen Z, He M, Zhang L, Yang L, Wei L. Recent advances in the role of mesenchymal stem cells as modulators in autoimmunity and inflammation. Front Immunol. 2024;15:1525380. doi:10.3389/fimmu.2024.1525380.
41. Li H, Bai L. Advances in mesenchymal stem cell and exosome-based therapies for aging and age-related diseases. Stem Cell Res Ther. 2025;16:401. doi:10.1186/s13287-025-04318-1.
42. U.S. Food and Drug Administration. FDA warns patients and health care professionals about exosome products. FDA; updated 2024. Accessed January 27, 2026.
43. Yang L, Zhan Y, Ruan H, Li H. Mechanisms and parameters of cryotherapy intervention for early postoperative swelling following total knee arthroplasty: A scoping review. J Exp Orthop. 2024;11:45. doi:10.1002/jeo2.70197. https://esskajournals.onlinelibrary.wiley.com/doi/full/10.1002/jeo2.70197.
44. Stanhope EN, Warnett RL, Burt DG, et al. The influence of circulating cold water cryotherapy with or without intermittent pneumatic compression on shoulder joint position sense (JPS) in recreationally active adults: A randomized crossover trial. J Bodywork Movement Ther. 2024;40:1008-1013. https://www.sciencedirect.com/science/article/pii/S1360859224003589.
45. Alayat MS, Battecha KH, Jabr YS, Zagzoog F, Hasaballah B, Alsulami FFS, Refaei MA, Almehmadi OS. The Effectiveness of Cryoflow Cooling on Forearm Skin Temperature and Nerve Conduction Velocity in Normal Subjects: A Case–Control Study. NeuroSci. 2026; 7(1):1. https://doi.org/10.3390/neurosci7010001
46. U.S. Food and Drug Administration. Whole body cryotherapy: can extreme cold improve your health? FDA Consumer Update; 2016. Accessed January 27, 2026.
47. Costello JT, et al. Whole-body cryotherapy for muscle soreness. Cochrane Database Syst Rev. 2015.
48. Lyu K, Liu X, Jiang L, Chen Y, Lu J, Zhu B, Liu X, Li Y, Wang D, Li S. The Functions and Mechanisms of Low-Level Laser Therapy in Tendon Repair (Review). Front Physiol. 2022 Feb 15;13:808374. doi: 10.3389/fphys.2022.808374. PMID: 35242050; PMCID: PMC8886125.
49. U.S. Food and Drug Administration. 510(k) Premarket Notification K051922: MLS Family diode laser. FDA; September 14, 2005. Accessed January 27, 2026.
50. Smallcomb M, Khandare S, Vidt ME, Simon JC. Therapeutic Ultrasound and Shockwave Therapy for Tendinopathy: A Narrative Review. Am J Phys Med Rehabil. 2022 Aug 1;101(8):801-807. doi: 10.1097/PHM.0000000000001894. Epub 2021 Oct 4. PMID: 35859290; PMCID: PMC9304757.
51. Longest Medical. Regulatory and FDA clearance guide for shockwave devices. 2025. Accessed January 28, 2026.
52. Schoenhaus Gold J. Pilot Study: Fat Pad Restoration With Adipose Allograft in Metatarsalgia Patients Shows Structural, Functional Improvement. Plast Reconstr Surg Glob Open. 2025 Dec 3;13(12):e7336. doi: 10.1097/GOX.0000000000007336. PMID: 41346924; PMCID: PMC12674145.
53. Bcharah G, Tummala SV, Braithwaite CL, Karsen PJ, Patel KA. Fat grafting for pedal fat pad atrophy: A narrative review of the literature. J Foot Ankle Surg. 2025 Jul 17:S1067-2516(25)00213-3. doi: 10.1053/j.jfas.2025.07.008. Epub ahead of print. PMID: 40683455.
54. U.S. Food and Drug Administration. Statement on FDA’s comprehensive policy framework for regenerative medicine. FDA. November 16, 2017. Updated. Accessed January 28, 2026. https://www.fda.gov/news-events/press-announcements/statement-fdas-comprehensive-policy-framework-regenerative-medicine
© 2026 HMP Global. All Rights Reserved.
Any views and opinions expressed are those of the author(s) and/or participants and do not necessarily reflect the views, policy, or position of Podiatry Today or HMP Global, their employees, and affiliates.