Outpatient Care Model for Teclistamab Step-Up Dosing for the Treatment of Relapsed or Refractory Multiple Myeloma at OneOncology: Insights From a Managed Network of Independent Physician-Owned Community-Based Oncology Practices
Abstract
To mitigate adverse event (AE) risk, teclistamab is initiated with step-up dosing (SUD); however, questions remain around enabling logistics for outpatient SUD care models to improve convenient and safe patient access to teclistamab. A semi-structured qualitative interview with the vice president of oncology pharmacy at OneOncology sought guidance for administering outpatient teclistamab SUD for community-based oncology practices. OneOncology developed a comprehensive playbook to support physician education on teclistamab administration and AE management. Guidance has evolved as network partners have gained familiarity with teclistamab, shifting from a hybrid SUD model (administering outpatient SUD and then admitting for observation) to also incorporating full outpatient SUD. Criteria for outpatient SUD implementation include caregiver access, ability to monitor vital signs, and proximity to the clinic site. The guidance is designed to be flexible for use across varying practices in the OneOncology network. Outpatient and hybrid SUD models for teclistamab are feasible and have been safely implemented in community-based settings. This playbook serves as an example to inform the development of future teclistamab care models.
Introduction
In October 2022, the US Food and Drug Administration (FDA) granted accelerated approval to the first B-cell maturation antigen (BCMA) directed CD3 T-cell engager, teclistamab, for the treatment of relapsed or refractory multiple myeloma (RRMM) among patients who had received at least four prior lines of therapy and were triple class exposed, based on results from the MajesTEC-1 study.1 Prior to teclistamab approval, this category of patients had a median overall survival (OS) of 12 months and a median progression-free survival (PFS) of 4 months, with a 40% overall response rate (ORR) to their first treatment with teclistamab. In the long-term analysis of the MajesTEC-1 trial, patients treated with teclistamab attained a median OS of 22.2 months, a median PFS of 11.4 months, and an ORR of 63%, with 46% of patients exhibiting a complete response or better.2 Similar response rates are observed in the real-world setting, with ORR between 59% and 66% reported in six studies of over 50 patients and at least 3 months of follow-up.3
Teclistamab has a unique adverse event (AE) profile, including risks of cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity (ICANS). To mitigate the risk and severity of CRS and ICANS, the FDA recommends step-up dosing (SUD), which gradually increases teclistamab doses to the target therapeutic dose, while patients are monitored in a hospital setting. The product label advises a 48-hour inpatient observation period after each of the three step-up doses.4
For both patients and health care systems, inpatient observation protocols are logistically complex and burdensome. Hospitalization requires the use of hospital resources, including medical personnel and beds, potentially limiting patient access in rural or underserved areas. As a result, some health care practices have shifted to hybrid or outpatient care models.5-7 Community-based oncology practices may particularly benefit from outpatient SUD models given the reduced reliance on hospital-based care. However, best practices for implementing outpatient models of care in community settings, including practice resource management, remote monitoring of AEs, and appropriate patient selection, are yet to be properly documented. Thus, there is an essential need to better understand current real-world practice insights from community-based oncology practices with experience in delivering outpatient teclistamab SUD.
OneOncology, a physician-driven managed service organization, aims to expand cost-effective community-based, patient-focused, and equitable access to novel cancer treatments throughout the US by supporting a network of independent physician-owned oncology practices. Specifically, OneOncology collaborates with community-based oncology practices and offers various tools to support value-based care. Recognizing the need for adoption of bispecific therapies among community-based practices to support the provision of cost-effective, patient-focused, and equitable access to care, OneOncology developed teclistamab SUD guidance that can be adapted to the unique needs and workflow of each clinical practice.
This report describes the OneOncology outpatient care model for teclistamab, including guidance and implementation strategies that are designed to be customized by their community-based network partners, including clinics in underserved communities (eg, rural or low-income settings). A semi-structured qualitative study was conducted to understand the development of OneOncology’s playbook for outpatient teclistamab SUD administration and AE management processes.
Methods
A semi-structured, in-depth interview was conducted with the oncology pharmacist who spearheaded the development of OneOncology’s teclistamab SUD playbook. The interview focused on the organization’s experience with structuring teclistamab SUD in both hybrid and outpatient models for affiliated practices that treat patients with RRMM. Discussion topics included SUD model history and evolution, barriers and challenges in initiating outpatient and hybrid SUD models, processes of partnering with local hospitals, and considerations for scalability. The interview was audio-recorded, transcribed verbatim, and analyzed using qualitative data analysis software. An a priori coding scheme was developed, and the transcript was independently coded by two members of the research team. The findings from this qualitative study are summarized descriptively by themes.
Results
Developing a Playbook for Teclistamab Administration
Shortly following FDA approval of teclistamab, the OneOncology pharmacy services team developed a playbook to support the successful implementation of a teclistamab SUD model in a hybrid outpatient/inpatient setting. OneOncology’s playbook was designed to allow for customization to accommodate the workflow needs and resource constraints of an individual practice. Informed by National Comprehensive Cancer Network® Guidelines, the playbook contains CRS and ICANS management guidance, prescribing information, clinical trial results, and workflow considerations. The playbook is an evolving document that provides best practice guidance on implementation of teclistamab SUD care models. Upon rollout in December 2022, the playbook recommended a hybrid SUD model, in which clinics administer each step-up dose in an outpatient setting and then admit the patient to a hospital or tertiary center for a 48-hour observation period following each dose, utilizing 3-day intervals between step-up doses (ie, a dosing schedule of days 1, 4, and 7). Additionally, the playbook provides network partners with a centralized educational resource on how to monitor, diagnose, and manage CRS and ICANS (Figure 1). Further, this resource details teclistamab administration and workflow considerations, including how to administer the drug, what measures need to be in place before drug administration (ie, Risk Evaluation and Mitigation Strategy [REMS] certification), and when a patient should begin the treatment cycle.
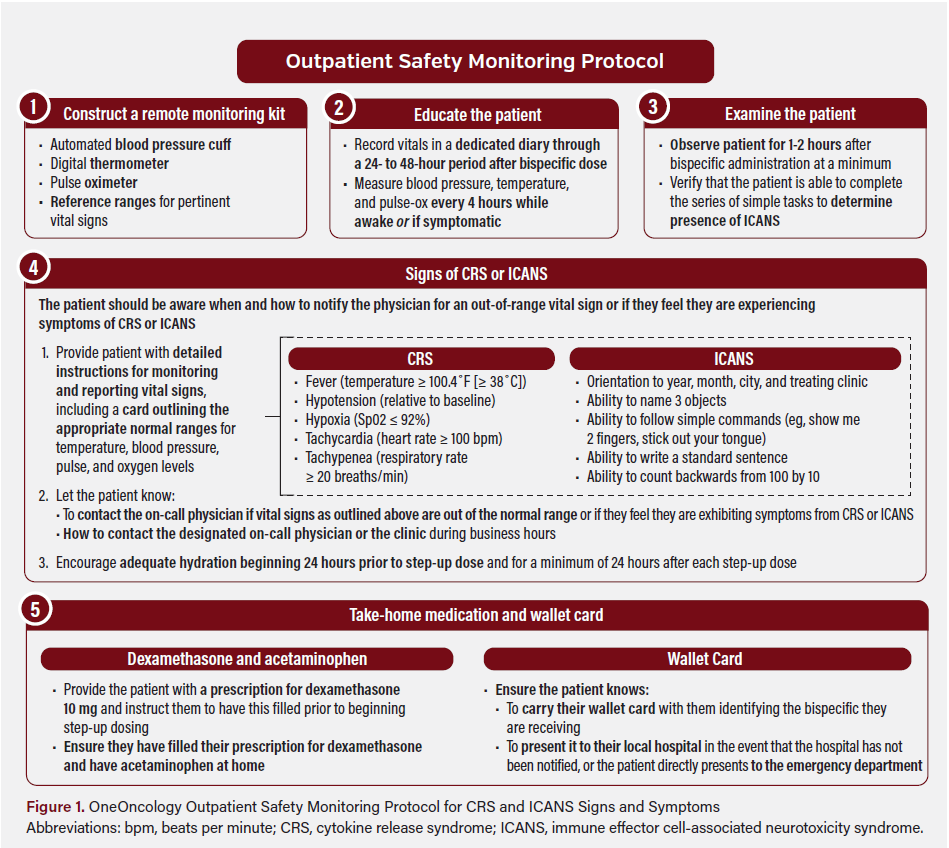
OneOncology also provides its network partners with a supplementary educational slide deck comprising data from guidelines, prescribing information, and pivotal trials on each bispecific therapy. The deck builds on existing REMS programs by offering detailed guidance on monitoring protocols, including timing for potential AEs. A virtual platform supports a peer-learning environment where practices can connect via group meetings and group chats to share information and resources on treatment administration approaches. Practices can communicate directly with one another about various aspects of care, including new research findings, clinical trial opportunities, and experiences and perspectives around implementing different care models. These educational and collaborative tools are designed to foster independence and growth, supporting physicians in delivering care within their local communities, including underserved communities, while navigating the complexities of cancer care, such as staying informed of new research and therapies and managing drug expenses and reimbursement formularies.
A Customizable Outpatient Model for Teclistamab Step-up Dosing
In April 2024, after observing an increase in comfort level and confidence among practices employing the hybrid care model, OneOncology added an outpatient care model for teclistamab SUD to the playbook. This model was developed to address the need for a broader care approach for teclistamab administration that aims to reduce hospital burden, health care costs, and the risk of infection, as well as improve the patient experience. With safety as the foremost priority (eg, AE prevention, monitoring, and timely hospital admission), the model was designed to allow clinics to pivot as needed and customize their care approach to support clinic workflow. Although the playbook does not list specific implementation requirements for physicians, it outlines the following recommendations for the clinical care teams (Figure 2):
- Evaluate: To establish confidence that treatment can be received safely in the community, the care team should evaluate the patient to confirm that an adequate support system is in place (eg, 24-hour caregiver) informed on the patient’s treatment course. Either the patient or their caregiver must be able to monitor the patient’s vital signs every 4 hours, and both must be aware of the abnormal vital signs that prompt immediate care. The patient must also be able to attend the clinic daily between step-up doses.
- Educate: To ensure that patients and their caregivers have the necessary knowledge to monitor and manage potential AEs, a member of the care team (ie, nurse, advanced practitioner, physician, or pharmacist) provides in-person education about the signs and symptoms of CRS, ICANS, and infection. Patients should receive wallet cards with information about their ongoing treatment with bispecific therapy. Patients should be given additional education on monitoring potential AEs and be advised on when to contact their physician (eg, if symptoms present), which on-call number to use, when to seek hospital care, and when to present their wallet card.
- Ensure: To support AE monitoring and management, the care team should ensure that the patient and caregiver have the necessary tools to monitor vital signs, including a blood pressure cuff, finger pulse oximeter, thermometer, and written diary to record vital sign information. Patients or caregivers should report vital sign values to the clinic each evening via telephone. In the event of a grade 1 CRS or mild infection, the care team should ensure patients have access to the necessary medications, including acetaminophen, dexamethasone, and antiviral medications. Intravenous immunoglobulin (IVIG) is given at the discretion of the provider, typically reserved for patients with immunoglobulin G (IgG) levels below 400 mg/dL.
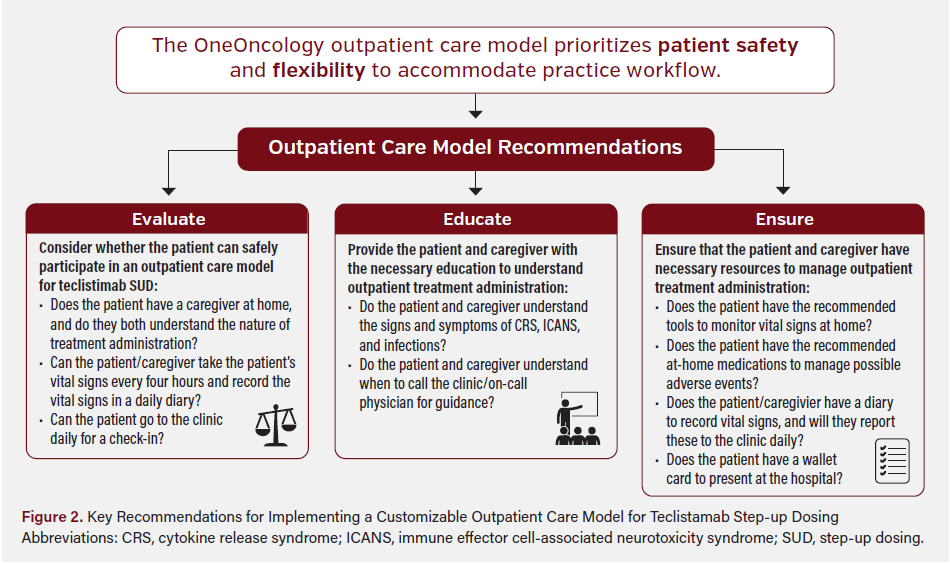
OneOncology has observed that the key to scaling outpatient teclistamab SUD care models in the community practice setting (ie, from a hybrid to outpatient care model) is clinical staff ’s gained knowledge of and experience with the safety and feasibility of a hybrid model. Once the required practice-hospital partnership and workflow are successfully established for a hybrid approach, staff comfort and confidence with teclistamab administration increase, thereby supporting adoption of a fully outpatient care model for teclistamab SUD.
Challenges and Future Care Model Considerations
To implement an outpatient (and hybrid) care model for teclistamab SUD, clinical practices must work with a hospital partner that directly admits patients (ie, bypass the emergency department [ED]) and maintains consistent access to tocilizumab to manage CRS. Potential hospital partners are often identified through existing relationships between community-based practices and local hospitals. Once a partner is identified, a main challenge to delivering an outpatient care model for teclistamab SUD is establishing a successful practice-hospital partnership. To overcome this challenge, OneOncology recommends the following:
- The practice-hospital partnership must share open communication and foster relationships between the clinic and hospital staff on relevant care units (ie, ED, intensive care unit, general oncology).
- Establish a streamlined pathway for patient care.
- Take a multifaceted educational approach, whereby the clinic educates patients about the wallet cards and importance of presenting them at the hospital. By doing so, the hospital partner is vested in educating all staff from relevant care units to ensure timely action when patients present their wallet card.
- Ensure the hospital partner has tocilizumab on site.
Community-based practices may leverage OneOncology’s resources and support to educate their staff. Education at the hospital-partner level, however, relies on coordinating with the life science companies that manufacture bispecific products.
Another challenge to adopting an outpatient care model is the financial risk associated with bispecific treatment administration. Clinical sites face the risk of delayed payment when using a J-code due to increased logistical complexities on the insurance company side. Payment rejections can occur if insurers identify issues with claim submissions, triggering costs associated with the time and resources needed to address them. Overcoming this challenge requires investment in staff training to efficiently resolve J-code rejections.
Hospitals also express concerns about reimbursement and receiving adequate payment for AE treatment costs, such as costs associated with tocilizumab, when a patient transitions from observation to inpatient status. Building provider confidence in the tolerability of bispecific antibody options and that mild CRS can be appropriately managed in the outpatient setting is essential to overcoming these concerns.
Finally, practices may face challenges unique to their practice model. To optimize resources, larger clinical practices with multiple sites may adopt a hub-and-spoke model, strategically designating specific clinics for teclistamab SUD administration. This approach maximizes patient access to safe treatment while facilitating hospital partnerships when needed.
The use of electronic monitoring services may further support the successful delivery of outpatient care. However, additional guidance is needed on implementing these services and navigating billing for remote monitoring.
To further support the adoption of an outpatient care model for bispecific treatment administration in community-based oncology practices, OneOncology advises involving these practices during drug development activities. Including these practices in clinical trials supports access to diverse patient populations (ie, rural or urban, as well as racially, ethnically, and socioeconomically diverse), and it supports a better understanding of treatment outcomes among these patient groups. Moreover, community-based oncology practices that participate in clinical trials will become more familiar and comfortable with administering these therapies when they become commercially available.
Conclusion
With recent advancements in bispecific antibody treatment for RRMM, there is a need to better understand real-world practice insights on current guidance, tools, resources, and strategies needed to safely implement an outpatient care model for teclistamab SUD. This treatment administration approach will contribute to reduced health care costs (eg, fewer hospital stays) and improved quality of cancer care by aligning patient preferences for clinic-based treatment and home monitoring.
In a recent retrospective study conducted within their network of practices, OneOncology reported that most patients completed outpatient SUD without hospitalization, and rates of CRS/ICANS were similar across the outpatient, inpatient, and hybrid cohorts.8 These findings support the safety and feasibility of outpatient and hybrid SUD models while demonstrating reduced health care resource utilization.
Key facilitators to implementing an outpatient care model for teclistamab SUD include an established practice-hospital partnership; provider experience, comfort, and confidence with hybrid care models; and provider proficiency in AE management. Managed service organizations like OneOncology play a vital role in building the capacity of community-based oncology practices to implement and manage new therapies, including bispecific antibody treatment administration. Such organizations can provide foundational information and resources, such as implementation playbooks, educational slide decks, and outpatient bispecific monitoring protocols, tailored to individual practice workflows. These organizations also offer a shared space that supports open communication and collaborative learning among network partners. Based on current insights, OneOncology envisions a broader uptake of outpatient care models for teclistamab SUD that prioritize patient safety. Their model provides a roadmap for interested community practices to educate and empower their physicians to safely implement outpatient or hybrid SUD administration. Ultimately, their approach benefits patients by enhancing access to care, particularly in underserved areas, and serves as a framework for future care models in similar settings.
Clinical Pathway Category: Infrastructure & Innovation
This study exemplifies infrastructure & innovation within clinical pathways by establishing a structured, scalable framework for implementing outpatient step-up dosing (SUD) of teclistamab in community oncology settings. Through the creation of OneOncology’s adaptable playbook, it aligns with evidence-based safety standards, enhances care accessibility, and supports consistent, high-quality delivery of oncology therapy across diverse practice environments.
Author Information
Authors: Lisa Raff, PharmD, MSPharm1; Rozanne Wilson, PhD2; Richard Murphy, BA2; Amal Jamaleddine, BA2; Nicole Bariahtaris, BA2; Meaghan Roach, MPH2
Affiliations: 1OneOncology, Nashville, TN; 2Precision AQ, New York, NY
Address correspondence to:
Lisa Raff, PharmD
OneOncology
Email: lisa.raff@oneoncology.com
Disclosures: L.R. has received speaker honoraria for Johnson & Johnson. R.W, R.M., A.J., and N.B. are employees of Precision AQ. M.R. is a former employee of Precision AQ.
References
1. FDA approves teclistamab-cqyv for relapsed or refractory multiple myeloma. US Food and Drug Administration. 2022. Accessed September 26, 2025. https://www. fda.gov/drugs/resources-information-approved-drugs/fda-approves-teclistamab-cqyv-relapsed-or-refractory-multiple-myeloma
2. Garfall A, Nooka A, van de Donk N, et al. Long-term follow-up from the phase 1/2 MajesTEC-1 trial of teclistamab in patients with relapsed/refractory multiple myeloma. J Clin Oncol. 2024;42(16)(suppl):7540. doi:10.1200/JCO.2024.42.16_suppl.7540
3. Derman B, Tan C, Steinfield I, et al. Real-world evidence evaluating teclistamab in patients with relapsed/refractory multiple myeloma: a systematic literature review. Cancers (Basel). 2025;17(7). doi:10.3390/cancers17071235
4. Moreau P, Garfall AL, van de Donk N, et al. Teclistamab in relapsed or refractory multiple myeloma. N Engl J Med. 2022;387(6):495-505. doi:10.1056/NEJMoa2203478
5. Tabbara N, Singel M, Allen N, et al. Ambulatory teclistamab administration in patients with relapsed/refractory multiple myeloma. J Clin Oncol. 2024;42(suppl 16):11146. doi:10.1200/JCO.2024.42.16_suppl.11146
6. Derman BA, Roach M, Lin D, et al. Panel interview of oncology practices with emergent experience of teclistamab in the real world: the TecPIONEER study. Curr Med Res Opin. 2024;40(6):1053-1058. doi:10.1080/03007995.2024.2352856
7. Varshavsky-Yanovsky AN, Styler M, Khanal R, Abdelmessieh P, Fung H. P940: An outpatient model for teclistamab step-up dosing administration—initial experiences at Fox Chase Cancer Center BMT program. Hemasphere. 2023;7(suppl). doi:10.1097/01.HS9.0000970664.60500.7f
8. Raff L, Abrams J, Barisonek C, et al. Outcomes of outpatient step-up dosing (SUD) of teclistamab and talquetamab in patients with relapsed/refractory multiple myeloma (RRMM): findings from a large network of community practices in the USA. Presented at: International Myeloma Society; September 2025; Toronto, Canada.














