Navigating the Management of Metastatic Colorectal Cancer in the Advanced Treatment Setting: Insights From an Expert Panel
Abstract
Mortality rates for metastatic colorectal cancer (mCRC) are increasing among those younger than 50 years of age. Two additional treatments were approved in 2023, with currently no recommendations for how to best sequence these available treatment options. Based on this, a panel of experts convened in April 2025 to discuss 1) considerations for selecting third-line and beyond (3L+) treatment for mCRC and 2) sequencing 3L+ treatments for mCRC. The group agreed that improved efficacy was the biggest unmet need, and that both patient-related factors (eg, functional age and level of social support) and disease-related factors (eg, actionable biomarkers and prior therapies) are important to treatment selection. Throughout the discussion, the panelists emphasized the importance of conducting a full molecular profile to guide treatment selection. The panelists typically use TAS-102 (trifluridine [FTD]/tipiracil [TPI]) plus bevacizumab first, followed by either regorafenib or fruquintinib, and recommended dose escalation or reduction strategies to manage adverse events. Additionally, the group agreed that there are several promising investigational targets for mCRC in addition to established treatment options.
Introduction
New cases of colorectal cancer (CRC) in 2025 are estimated to exceed 150 000 in the US.1 And, although the overall incidence has been declining among individuals over age 65, rates have been increasing in people aged 50 years and younger, which has historically been the unscreened population.2 Additionally, mortality rates have increased by 1% annually in people younger than 50 years of age since the mid-2000s.2 The 5-year survival rate for localized CRC is more than 90%; however, 1 in 5 patients with CRC present with de novo metastatic disease (stage IV) at diagnosis, and 5-year survival rates for metastatic CRC (mCRC) are only 16%.1 Moreover, an additional 40% of patients initially diagnosed with stage II or III CRC are at risk of progressing to metastatic disease.3 With improvement in treatment outcomes, a larger proportion of patients are reaching the third-line and beyond (3L+) treatment stage, emphasizing the need for effective treatment options for mCRC.
Regorafenib—a small molecule multi-kinase inhibitor targeting multiple kinases such as RET, RAF1, BRAF, and vascular endothelial growth factor (VEGF)—was approved in 2012 for mCRC previously treated with chemotherapy (including fluoropyrimidine, oxaliplatin, and irinotecan) and available biologic therapies—eg, an epidermal growth factor receptor (EGFR) inhibitor (if RAS wild-type [WT]) or a VEGF inhibitor (Table).4 In the CORRECT trial, regorafenib plus best supportive care (BSC) showed statistically significant improvements in overall survival (OS) and progression-free survival (PFS) vs placebo.5 The most common adverse events (AEs) (any grade) were fatigue, hand-foot skin reaction (HFSR), and diarrhea (Table).5 TAS-102 (trifluridine [FTD]/tipiracil [TPI]) is an oral chemotherapy combination of trifluridine (a thymidine-based nucleoside analog) and tipiracil (a thymidine phosphorylase inhibitor) approved in 2015 for patients with mCRC who had previously received fluoropyrimidine-, oxaliplatin-, and irinotecan-based chemotherapy; a VEGF inhibitor; and, if RAS WT, an anti-EGFR therapy.6 The RECOURSE study showed statistically significant improvement in OS and PFS with FTD/TPI plus BSC compared with placebo plus BSC (approximately 20% of patients had received prior regorafenib), and the most common AEs were nausea, vomiting, and decreased appetite (Table).7 In 2023, FTD/TPI plus bevacizumab was approved for the same indication as FTD/TPI monotherapy.6,8 In the SUNLIGHT trial, there were statistically significant improvements in OS and PFS vs FTD/TPI monotherapy, and the most common AEs were neutropenia, nausea, and anemia.9 Also approved in 2023 was fruquintinib, a small-molecule VEGF inhibitor approved for the treatment of patients with mCRC previously treated with fluoropyrimidine-, oxaliplatin-, and irinotecan-based chemotherapy; an anti-VEGF therapy; and, if RAS WT and medically appropriate, an anti-EGFR therapy.10 The FRESCO-2 trial showed statistically significant improvements in OS and PFS with fruquintinib plus BSC vs placebo plus BSC.11 The most common AEs were hypertension, asthenia, and decreased appetite.11 While studied in the fourth line in patients previously exposed to regorafenib and/or trifluridine-tipiracil, the US Food and Drug Administration (FDA) approval does not require patients to have been treated with one of these agents before use of fruquintinib.
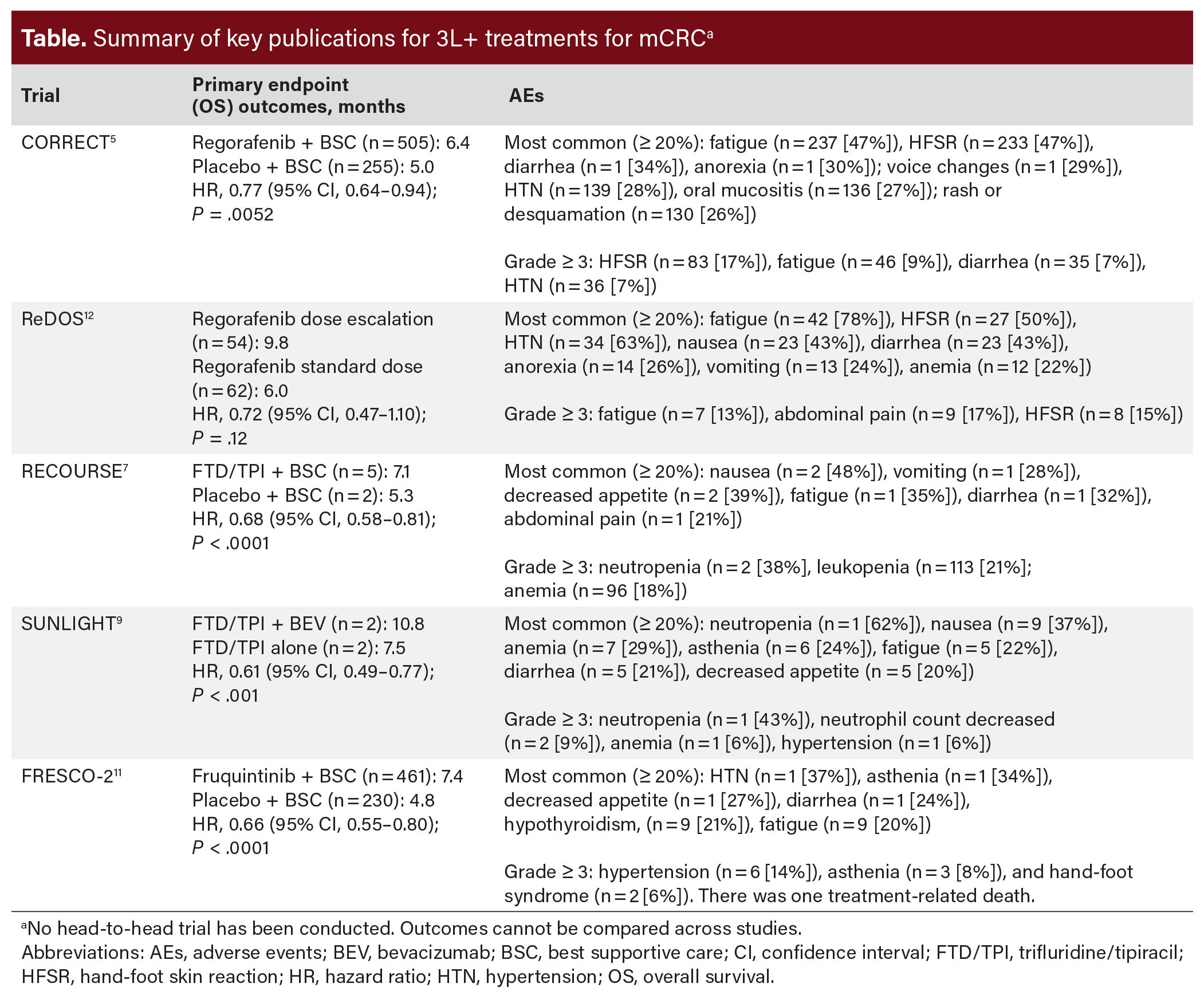
When selecting the best treatment approach for patients for whom the disease has progressed on at least 2 lines of therapy, there are several factors to consider. As these patients tend to be sicker, patient characteristics such as age, performance status (PS), and prior medical history are important considerations.13 Disease-specific factors, such as biomarkers and prior treatments, also play a key role in treatment decisions.13 The FDA-approved therapies in this advanced treatment setting for mCRC differ in their mechanisms of action, efficacy, and toxicity profile (Table). Although a wealth of clinical trial data and real-world evidence exists for these treatments, the National Comprehensive Cancer Center (NCCN) Clinical Practice Guidelines in Oncology (NCCN Guidelines®) do not specify the order in which the treatments should be given.14 An expert panel of clinicians was convened to: 1) understand the role of patient characteristics and disease-specific factors in selecting 3L+ treatment for mCRC; and 2) gain insight into approaches to sequencing FDA-approved 3L+ treatments for mCRC. This article summarizes the perspectives and insights gained from this discussion.
Methods
The expert panel discussion took place in April 2025 and allowed clinicians to share their expertise and knowledge about pertinent topics related to mCRC in the advanced treatment setting. The 2-hour discussion included a moderator (Dr Bekaii-Saab) and 3 US-based clinicians. The discussion was centered around 2 themes: 1) considerations for selecting 3L+ treatment for mCRC and 2) sequencing of FDA-approved 3L+ treatments for mCRC. Supporting materials to stimulate discussion were distributed before the meeting. These materials included an outline of discussion topics, published articles about relevant issues such as sequencing, cost, and real-world evidence, and published clinical trial results for the approved treatment options for 3L+ mCRC. The discussion was recorded.
Results
Considerations for Selecting 3L+ Treatment for mCRC
As part of the discussion for treatment selection, the group discussed current unmet needs in 3L+ mCRC. The group agreed that treatment efficacy was the greatest challenge (eg, for those treated with FTD/TPI plus bevacizumab who had received prior bevacizumab, the PFS was only 4.5 months).9 The discussion also identified challenges for treating select patients with peritoneal disease and bowel obstructions, because oral (pill-based) treatment regimens are harder to tolerate. The panelists noted that the current treatment options for 3L+ mCRC all include anti-angiogenic components. The group emphasized the need to avoid agents that target the angiogenic pathway in patients at risk for or in the presence of bleeding, bowel obstruction, arterial thrombotic events, etc.
The group also discussed other factors and considerations, specifically patient characteristics, when selecting treatments for refractory mCRC. The panelists noted that bone marrow function is important, especially when considering a cytotoxic agent such as TAS-102. In terms of myelosuppression, whether or not the patient has experienced myelosuppression in the past and recovered should be considered, as it may indicate the need for dosing regimen adjustments. The current platelet count is also important. For example, factors such as clinically significant or severe anemia and whether the patient is transfusion-dependent are important to consider.
Patient preferences, comorbidities, performance status, drug toxicity profile, and presence of residual toxicity from prior therapies are also factors to consider when selecting a 3L+ regimen. With that in mind and regarding the patient’s age, the panelists considered the patient’s functional age to be more useful than the numerical age when selecting appropriate treatments. Functional status and the presence of a social support system should also be a consideration. Health literacy and education are also crucial as they affect the patient’s ability to take oral medications as directed.
The discussion also identified disease-specific factors, such as biomarkers and prior therapies, when selecting treatment. Throughout the discussion, participants emphasized the importance of conducting a full molecular profile for biomarkers such as mismatch/repair (MMR) status, human epidermal growth factor receptor 2 (HER-2) amplifications, RAS mutations (KRAS or NRAS), and BRAF V600E mutations. This profile should be performed early to determine appropriate treatment options for first and second lines of therapy. The group also emphasized the importance of sidedness when selecting treatment. For example, for a patient with RAS WT disease with a right-sided tumor, EGFR-targeted therapy is not a recommended option. EGFR rechallenge can be considered for select patients in the 3L+ setting, especially those with a robust response and tolerability to initial EGFR therapy. The interval from the last EGFR dose should also be considered; one panelist recommended waiting at least 6 months before rechallenging. Participants shared that confirming RAS activity status via liquid biopsy may also help implement this EGFR rechallenge strategy.
"I utilize the ReDOS schedule—I think it makes things a lot easier for the patients." —Dr Marwan Fakih
For currently approved 3L+ agents, panelists discussed strategies for managing AEs. To manage AEs with regorafenib, all panelists utilize the dose escalation strategy outlined in the randomized, phase 2 ReDOS study.12 Results from the study showed that a dose escalation strategy of an 80 mg/day starting dose, 120 mg/day for Week 2, and the full dose of 160 mg/day for Week 3 as tolerated, had comparable efficacy and a lower incidence of AEs compared with starting at a dose of 160 mg/day. The group agreed that this strategy works in their practice, and many patients can achieve the full 160 mg/day dose.
For fruquintinib, 2 panelists shared that they start at 5 mg and deescalate the dose as needed. The group noted that fatigue is an issue but improves with dose reductions. For FTD/TPI plus bevacizumab, the panelists follow the 2 weeks on-treatment and 2 weeks off-treatment dosing regimen outlined in the package insert. One panelist shared that when adverse events occur, the patient is switched to a “1 week on-treatment, 1 week off-treatment” regimen. However, some patients do not feel better even after switching to this regimen, as the adverse effects may be due to the drug itself rather than the dosing schedule. One panelist noted that bone marrow suppression is the biggest concern with FTD/TPI plus bevacizumab and that granulocyte colony-stimulating factor (G-CSF) support and dose reduction may be needed.
"Where I think real-world data can be really helpful is in the less perfect patient, you know, such as mild or moderate liver issues, or mild or moderate renal issues, where they often would have been excluded from trials, but we may still choose to treat them." —Dr Stacey Cohen
Sequencing of FDA-Approved 3L+ Treatments for mCRC
The panelists do not follow clinical pathways at their institutions that recommend or suggest a specific sequence for the 3L+ treatments. Participants emphasized that caution should be applied for all FDA-approved agents, as outcomes may differ in the real-world patient population, including adverse events. Regarding sequencing, all panelists prefer to start with FTD/TPI plus bevacizumab except in situations where patient preference is to forego bevacizumab. Two panelists use fruquintinib next, followed by regorafenib; one panelist uses regorafenib first, followed by fruquintinib, citing real-world data for this choice.15 One participant stated that real-world data are helpful in treatment selection and sequencing as they can shed light on a drug’s efficacy and toxicity in patients outside of the clinical trial setting.
The group outlined the reasons for using FTD/TPI plus bevacizumab before the other 3L+ therapies. One panelist decides based on longer PFS and the level of comfort with the combination, noting that hematologic toxicities such as leukopenia observed with FTD/TPI plus bevacizumab do not significantly impact patient quality of life. However, the group agreed that for patients with severe thrombocytopenia or for whom bevacizumab or tyrosine kinase inhibitors (TKIs) in general are contraindicated, FTD/TPI plus bevacizumab would not be an appropriate treatment option. For patients who received chemotherapy as a second-line treatment and express interest in wanting a “chemotherapy break,” regorafenib or fruquintinib may be considered.
Participants shared additional considerations when treating with FTD/TPI plus bevacizumab. They noted that febrile neutropenia can occur, and as with any treatment, if the patient does not have a strong care partner support system, FTD/TPI plus bevacizumab may not be an appropriate option.
The group also discussed promising topics for future research. Participants noted that the established RAS inhibitors provide fairly robust outcomes (PFS of 5 to 6 months and overall response rate [ORR] of 30%-35%) in patients with KRAS G12C mutations, and it is of interest whether new RAS inhibitors would provide the same level of efficacy. Other areas of interest for future research included pan-KRAS inhibitors, regulating the RAS protein activation cycle, and the potential role of dual EGFR and MET inhibition. One panelist noted interest in the efficacy of combining regorafenib with an immunotherapy such as nivolumab in patients with microsatellite stable (MSS) mCRC without liver metastases. The durable responses observed with regorafenib, ipilimumab, and nivolumab for some patients with MSS CRC and progression on prior chemotherapy appear promising.16 There was additional excitement about the efficacy data for next-generation CTLA4 inhibitors in patients with CRC without liver metastases.17 Another area for future research is identifying the subgroups of patients who see greater benefits with specific therapies, such as those with lung and lymph node metastases.
Discussion/Recommendations
The panelists identified several unmet needs in the advanced treatment setting of mCRC. Therapies with more robust survival outcomes were identified as the greatest unmet need. Additionally, there is a need for therapies that target pathways other than the angiogenesis pathway. When selecting treatment, both patient-specific and disease-specific factors are important considerations. In addition to factors such as functional age, PS, and the level of a social support system, the panelists emphasized the importance of obtaining a full molecular profile to allow for a fully informed treatment plan. Patient preference was also discussed, specifically a patient’s request for a chemotherapy break.
Inherent with any cancer treatment, especially treatments among patients who tend to be older and have poorer functional status, 3L+ treatment options have AEs. Both neutropenia and myelosuppression were regarded as AEs of concern and could potentially lead to hospitalization; however, less severe cases were deemed manageable. The panel discussion highlighted the need to employ dosing strategies to reduce toxicities. For regorafenib, NCCN Guidelines® recommend use of the dose-escalation strategy from the ReDOS trial as an “appropriate alternative approach” for dosing.14 This dose escalation approach showed lower rates of AEs compared to the standard dosing group (160 mg/day starting dose).12 For fruquintinib, a dose reduction strategy is used, and for FTD/TPI ± bevacizumab, some panelists move to a 1-week-on, 1-week-off dosing regimen after starting with the recommended 2-weeks-on, 2-weeks-off regimen.
"I like the ReDOS schedule. I hardly ever have patients where I start with the full dose." —Dr Anwaar Saeed
The current NCCN Guidelines for the 3L+ setting do not offer specific guidance about the order in which to use each treatment.14 The NCCN Guidelines recommend FTD/TPI ± bevacizumab as an option for patients experiencing disease progression on prior standard therapies.14 Fruquintinib is recommended as an option for patients with previously treated mCRC who have experienced progression using all available regimens.14 Regorafenib is recommended as an option for patients with mCRC who are refractory to chemotherapy and can be given before or after FTD/TPI, FTD/TPI plus bevacizumab, or fruquintinib.14 Despite the absence of head-to-head clinical trial sequencing data in the 3L+ setting, there is real-world evidence showing statistically significantly prolonged survival with the use of regorafenib before fruquintinib and numerically longer survival as well as reduced neutropenia and myelosuppression with the use of regorafenib before FTD/TPI ± bevacizumab.15,18 The panelists’ collective opinion was that, although important, real-world evidence does not guide all clinical practice decisions, and there is a need for head-to-head clinical studies assessing the optimal treatment sequence. The group for most patients uses FTD/TPI plus bevacizumab first, followed by either regorafenib or fruquintinib.
Several trials are investigating new compounds and combination therapies for mCRC for patients who have progressed on previous lines of treatment. Immune checkpoint inhibitor combinations with anti-CTLA4 therapies in MSS mCRC tumors have shown survival outcome benefits, with the greatest benefits seen in patients without liver metastases.17 For example, promising results have been observed in a small Phase 1 trial assessing regorafenib, ipilimumab, and nivolumab for patients with MSS CRC and progression with prior chemotherapy.16 Data from combining cabozantinib, a VEGF and MET multikinase inhibitor, with PD-L1 inhibitors in the MSS chemorefractory CRC population have also shown promising outcomes and have led to the Phase III STELLAR-303 trial assessing the combination of zanzalintinib, a VEGF and MET multikinase inhibitor, with atezolizumab.19,20 KRAS and pan-KRAS inhibitors also show promise, as well as BRAF-targeted therapies combined with immune checkpoint inhibitors for treating BRAF V600E-mutant CRC.21
These findings have several limitations. The topic and discussion were subject to potential bias from the panelists and/or moderator. The discussion included insights based on the opinions of a group of clinicians, which may not apply or be generalizable to larger contexts. This was a small panel group, so viewpoints and insights may not represent diverse populations.
Conclusion
The mCRC treatment landscape for 3L+ treatments continues to expand. Treatment options are selected based on patient and disease-specific factors. Overall survival benefits for this heavily treated population remain an unmet need. Flexible dosing strategies can be used to address the side effects of treatment. Future directions include the emerging role of RAS inhibitors, other targeted therapies, and investigational immunotherapy combinations. Overall, the group agreed that access to all available treatment options is required to make the best individual treatment choices for patients.
Author Information
Affiliations:
1Department of Hematology and Medical Oncology, Mayo Clinic, Phoenix, AZ; 2Fred Hutchinson Cancer Center, Seattle, WA; Division of Hematology/Oncology, University of Washington, Seattle, WA; 3City of Hope Comprehensive Cancer Center, Duarte, CA; 4Division of Hematology/Oncology, Department of Medicine, University of Pittsburgh (UPMC), Pittsburgh, PA
Funding:
Bayer provided financial support for this activity. The participants were compensated for their time.
Correspondence:
Tanios Bekaii-Saab, MD
Mayo Clinic, Phoenix, AZ
Email: bekaii-saab.tanios@mayo.edu
Acknowledgments:
Medical writing support provided by HMP Collective.
Disclosures:
T.S.: has received institutional research funding from Agios, Arys, Arcus, Atreca, Boston Biomedical, Bayer, Eisai, Celgene, Lilly, Ipsen, Clovis, Seattle Genetics, Genentech, Novartis, Mirati, Merus, Abgenomics, Incyte, Pfizer, and BMS; has received consulting fees to his institution from Servier, Ipsen, Arcus, Pfizer, Seattle Genetics, Bayer, Genentech, Incyte, Eisai, Merus, Merck KGaA, and Merck, and to self from Stemline, AbbVie, Blueprint Medicines, Boehringer Ingelheim, Janssen, Daiichi Sankyo, Natera, Treos Bio, Celularity, Caladrius Biosciences, Exact Science, Sobi, BeiGene, Kanaph, AstraZeneca, Deciphera, Zai Labs, Exelixis, Elevar, Illumina, Foundation Medicine, Sanofi, GlaxoSmithKline, and Xilio; has served on independent data monitoring committees or data safety monitoring boards for The Valley Hospital, FibroGen, Suzhou Kintor, AstraZeneca, Exelixis, Merck/Eisai, PanCan, and 1Globe; has served on scientific advisory boards for Imugene, Immuneering, Xilis, Replimune, Artiva, and Sun Biopharma; has received royalties from UpToDate; and holds patents licensed to Imugene and Recursion.
S.C.: has received consulting fees to self from Pfizer, Taiho, Bayer, Regeneron, Eisai, Delcath, Isofol, Agenus, Exact Sciences, Guardant, Merck, and AbbVie; has received nonfinancial support from Biomea; and served on independent data monitoring committees for GlaxoSmithKline; has received institutional research funding from Biomea, Pfizer, BioNTech, and Tempus.
M.F.: has received institutional research funding from Amgen, Bristol Myers Squibb, Genentech, Verastem, and Novartis; has received consulting fees from AstraZeneca, Bristol Myers Squibb, Incyte, PsiOxus, Zhuhai Yufan Biotech, and Taiho Pharmaceutical; has received honoraria from Guardant360; and holds advisory roles from Amgen, Bayer, Array Biopharma, Eisai, GlaxoSmithKline, Merck, Mirati, Nouscom, Roche/Genentech, and Xenthera.
A.S.: has received institutional research funding from AstraZeneca, Bristol Myers Squibb, Merck, Clovis, Exelixis, Actuate Therapeutics, Incyte Corporation, Daiichi Sankyo, Five Prime Therapeutics, Oxford Biotherapeutics, Arcus Therapeutics, Amgen, Innovent Biologics, Dragonfly Therapeutics, KAHR Medical, and BioNTech; and has received advisory board fees from AstraZeneca, Bristol Myers Squibb, Exelixis, Pfizer, Xilio Therapeutics, Taiho, Amgen, Autem Therapeutics, KAHR Medical, and Daiichi Sankyo.
References
-
Cancer stat facts: colorectal cancer. National Cancer Institute. https://seer.cancer.gov/statfacts/html/colorect.html
-
Colorectal cancer facts & figures 2023-2025. American Cancer Society. Published 2023. Accessed June 5, 2025. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/colorectal-cancer-facts-and-figures/colorectal-cancer-facts-and-figures-2023.pdf
-
Sayagués JM, Montero JC, Jiménez-Pérez A, et al. Analysis of circulating tumor DNA in synchronous metastatic colorectal cancer at diagnosis predicts overall patient survival. Int J Mol Sci. 2023;24(9):8438. doi:10.3390/ijms24098438
-
Stivarga (regorafenib) tablets, oral. Full prescribing information. 2025.
-
Grothey A, Van Cutsem E, Sobrero A, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381(9863):303-312. doi:10.1016/S0140-6736(12)61900-X
-
Lonsurf (trifluridine and tipiracil) tablets, for oral use. Full prescribing information. 2023.
-
Mayer RJ, Van Cutsem E, Falcone A, et al. RECOURSE Study Group. Randomized trial of TAS-102 for refractory metastatic colorectal cancer. N Engl J Med. 2015;372(20):1909-1919. doi:10.1056/NEJMoa1414325
-
US Food and Drug Administration. FDA approves trifluridine and tipiracil with bevacizumab for previously treated metastatic colorectal cancer. US Food and Drug Administration. Published August 2, 2023. Accessed June 5, 2025. https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-trifluridine-and-tipiracil-bevacizumab-previously-treated-metastatic-colorectal-cancer
-
Prager GW, Taieb J, Fakih M, et al. Trifluridine-tipiracil and bevacizumab in refractory metastatic colorectal cancer. N Engl J Med. 2023;388(18):1657-1667. doi:10.1056/NEJMoa2214963
-
Fruzaqla. Prescribing information. Takeda Pharmaceuticals; 2023.
-
Dasari A, Lonardi S, Garcia-Carbonero R, et al. Fruquintinib versus placebo in patients with refractory metastatic colorectal cancer (FRESCO-2): an international, multicentre, randomised, double-blind, phase 3 study. Lancet. 2023;402(10395):41-53. doi:10.1016/S0140-6736(23)00772-9
-
Bekaii-Saab TS, Ou FS, Ahn DH, et al. Regorafenib dose-optimisation in patients with refractory metastatic colorectal cancer (ReDOS): a randomised, multicentre, open-label, phase 2 study. Lancet Oncol. 2019;20(8):1070-1082. doi:10.1016/S1470-2045(19)30272-4
-
Bekaii-Saab T, Kim R, Kim TW, et al. Third- or later-line therapy for metastatic colorectal cancer: reviewing best practice. Clin Colorectal Cancer. 2019;18(1):e117-e129. doi:10.1016/j.clcc.2018.11.002
-
National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®): Colon Cancer. V.1.2024. National Comprehensive Cancer Network. Published January 2024. https://www.nccn.org. Accessed June 5, 2025.
-
Zhang Q, Chen M, Wang Z, et al. Efficacy and safety comparison of regorafenib and fruquintinib in metastatic colorectal cancer—an observational cohort study in the real world. Clin Colorectal Cancer. 2022;21(3):e152-e161. doi:10.1016/j.clcc.2022.01.007
-
Fakih M, Sandhu J, Lim D, Li X, Li S, Wang C. Regorafenib, ipilimumab, and nivolumab for patients with microsatellite stable colorectal cancer and disease progression with prior chemotherapy: a phase 1 nonrandomized clinical trial. JAMA Oncol. 2023;9(5):627-634. doi:10.1001/jamaoncol.2022.7845
-
Bullock AJ, Schlechter BL, Fakih MG, et al. Botensilimab plus balstilimab in relapsed/refractory microsatellite stable metastatic colorectal cancer: a phase 1 trial. Nat Med. 2024;30(9):2558-2567. doi:10.1038/s41591-024-03083-7
-
Ahn DH, Bekaii-Saab TS, Yuan C, et al. Sequential treatment with regorafenib and trifluridine/tipiracil ± bevacizumab in refractory metastatic colorectal cancer in community clinical practice in the USA. Cancers (Basel). 2025;17(6):969. doi:10.3390/cancers17060969
-
Saeed A, Park R, Pathak H, et al. Clinical and biomarker results from a phase II trial of combined cabozantinib and durvalumab in patients with chemotherapy-refractory colorectal cancer (CRC): CAMILLA CRC cohort. Nat Commun. 2024;15(1):1533.
-
Saeed A, Tabernero J, Parikh A, et al. STELLAR-303: randomized phase III study of zanzalintinib + atezolizumab in previously treated metastatic colorectal cancer. Future Oncol. 2024;20(24):1733-1743.
-
Ciracì P, Studiale V, Taravella A, Antoniotti C, Cremolini C. Late-line options for patients with metastatic colorectal cancer: a review and evidence-based algorithm. Nat Rev Clin Oncol. 2025;22(1):28-45. doi:10.1038/s41571-024-00965-0
















