Decision Support Documentation for Targeted Therapy Eligibility in Breast Cancer
Background
The US Oncology Network’s clinical decision support system, Clear Value Plus (CVP), guides providers toward evidence-based treatment options for breast cancer, including prompts for biomarker documentation. In late 2023, the addition of capivasertib to the National Comprehensive Cancer Network (NCCN) Breast Cancer Guidelines introduced testing for AKT1 and PTEN alterations, alongside PIK3CA, into the treatment paradigm for patients with hormone receptor–positive, HER2-negative breast cancer following progression on prior endocrine therapy.
Although multiple targeted agents exist for PIK3CA-mutated disease, the presence of a mutation does not necessitate treatment with a targeted agent. This analysis evaluated the frequency of biomarker documentation in CVP, with a focus on changes from unknown to known molecular status after CVP re-prompted providers during subsequent treatment decisions.
Methods
A retrospective analysis was conducted using biomarker data from iKnowMed, an electronic health record used by more than 2700 providers across 600 US Oncology Network sites, from December 11, 2023, through June 30, 2025. Beginning in September 2024, CVP introduced re-prompts for providers to document biomarker results when previous values were listed as unknown. Testing results for AKT1, PTEN, and PIK3CA, along with corresponding treatment selections, were collected. No additional clinical outcomes were assessed.
Results
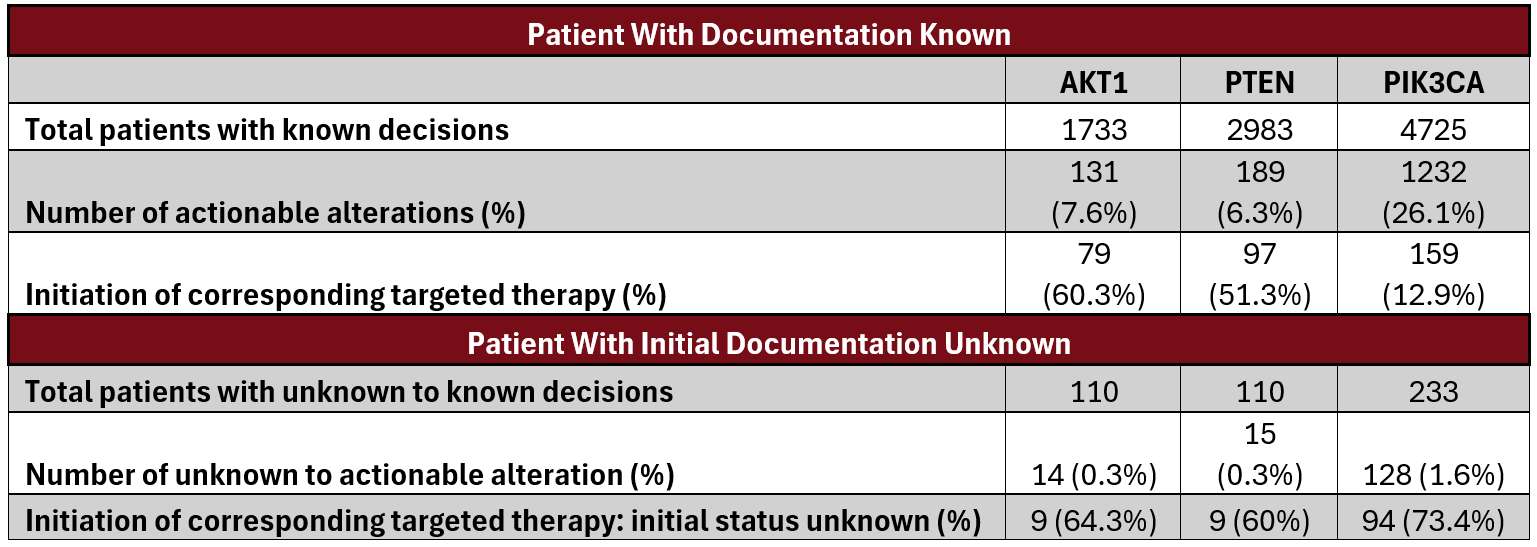
Among patients with documented biomarker results, alterations were detected in 131 of 1733 (7.6%) for AKT1, 189 of 2983 (6.3%) for PTEN, and 1232 of 4725 (26.1%) for PIK3CA. Following initial documentation of unknown status, actionable alterations were identified in 14 patients (0.3%) for AKT1, 15 (0.3%) for PTEN, and 128 (1.6%) for PIK3CA.
In this subset, targeted therapy was initiated in 9 of 14 (64.3%) AKT1, 9 of 15 (60.0%) PTEN, and 94 of 128 (73.4%) PIK3CA alteration–positive cases. Re-prompting led to a 33.4% increase in precision therapy selection for agents targeting the PI3K pathway.
Conclusion
Re-prompting providers within the CVP CDS tool was associated with improved biomarker documentation and increased use of precision therapies. These findings suggest that structured, integrated prompts support guideline-concordant treatment planning and help ensure timely identification of actionable alterations.
Prevalence rates for AKT1, PTEN, and PIK3CA alterations were consistent with prior reports, including those by Tao et al. Streamlined biomarker documentation workflows may enhance personalized treatment selection and improve the clinical utility of CDS tools in oncology.
Author Information
Authors:
Daniel Rubin, PharmD, BCOP1; Victoria Handy, PharmD, BCOP1; Steven Gilmore, PharmD, BCOP1; Stephen Clark, PharmD, BCOP1; Aimee Ginsburg, PharmD, BCPS1
Affiliation:
1The US Oncology Network/McKesson
References
- NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) for Breast Cancer V.5.2023. Published June 2023. Accessed December 5, 2023.
- Tao J, Sisoudiya S, Sivakumar S, Sokol E, et al. Abstract P5-02-20: Clinicogenomic landscape and function of PIK3CA, AKT1, and PTEN mutations in breast cancer. Clin Cancer Res. 2025; 31(12_Supplement): P5-02-20. doi: 10.1158/1557-3265.SABCS24-P5-02-20