Assessing EHR-to-EDC Data Transfer Solutions
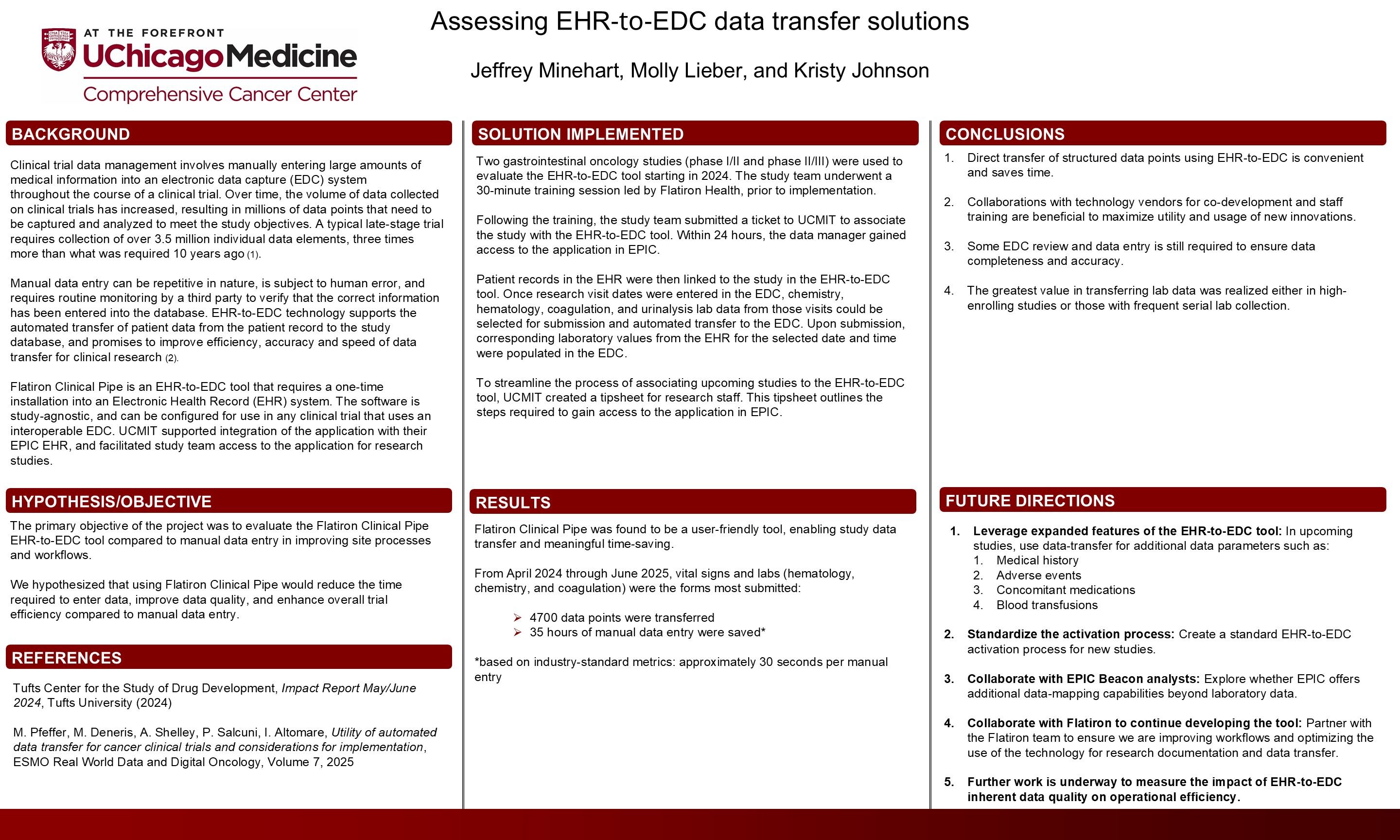
Background
Clinical trial data management traditionally involves manual entry into an electronic data capture (EDC) system. As clinical trial data volume increases, so does the burden of entering and verifying millions of data points. A typical late-stage trial now requires the collection of more than 3.5 million individual data elements—approximately 3 times the volume collected a decade ago.¹
Manual data entry is repetitive, time-consuming, prone to human error, and requires third-party monitoring for validation. Electronic health record (EHR)–to-EDC technology automates the transfer of structured patient data from the medical record to the study database and offers potential improvements in data accuracy, efficiency, and speed.²
Flatiron Clinical Pipe, an EHR-to-EDC integration tool, requires a one-time installation within an EHR system. The software is study-agnostic and can be configured for any clinical trial using an interoperable EDC. The University of Chicago’s UChicago Medicine IT (UCMIT) supported integration and provided access for participating research teams.
This study evaluated Flatiron Clinical Pipe’s automated data transfer capabilities relative to manual data entry, with the hypothesis that the tool would reduce data entry time, improve data quality, and enhance workflow efficiency.
Methods
Two gastrointestinal oncology studies (phase I/II and phase II/III) piloted use of the EHR-to-EDC tool in 2024. Before implementation, study teams participated in a 30-minute vendor-led training session conducted by Flatiron Health. Following training, teams worked with UCMIT to enable access within the Epic EHR and link patient records.
Entry of research visit dates into the EDC triggered the automatic transfer of corresponding laboratory data—including chemistry, hematology, coagulation, and urinalysis—from the EHR into the EDC.
Results
Flatiron Clinical Pipe was reported to be user-friendly and effective in enabling structured data transfer. From April 2024 to June 2025, the most commonly transferred data elements were vital signs and laboratory values. A total of 4700 data points were transferred, saving an estimated 35 hours of manual data entry, based on an industry standard of 30 seconds per entry.
Conclusion
Automated EHR-to-EDC transfer of structured data such as laboratory values offers time-saving and operational efficiencies. Manual review of EDC entries remains necessary to ensure data completeness and context.
The greatest efficiency gains were observed in high-enrolling studies and studies with frequent laboratory collections. Expanding EHR-to-EDC functionality to support the transfer of unstructured data—such as medical history, adverse events, concomitant medications, and blood transfusions—may further improve trial efficiency.
Future goals include standardizing the activation process for new studies, piloting additional data parameters, and collaborating with Epic Beacon analysts to explore enhanced data-mapping capabilities. Continued partnership with Flatiron Health will be essential to refine the tool and optimize workflows for research documentation and clinical trial data management.
Author Information
Authors:
Kristy L. Johnson1; Jeffrey Minehart1; Molly Lieber2
Affiliation:
1University of Chicago; 2Flatiron Health
References
- Tufts Center for the Study of Drug Development. May/June 2024 Impact Report. Tufts University; 2024. Accessed August 22, 2025. https://csdd.tufts.edu/publications/impact-reports
- Pfeffer M, Deneris, A M, Salcuni S P, Altomare I. Utility of automated data transfer for cancer clinical trials and considerations for implementation. ESMO Real World Data Digit Oncol. 2025; 7. doi: 10.1016/j.esmorw.2025.100112.