From Approval to Action: Evaluating Review Timelines for Oncology Therapies in Value Pathways
Background
Timely review of newly approved oncology therapies for inclusion in clinical pathways is critical to ensure that providers have access to the most current evidence-based treatment options. The US Oncology Network’s Pathways Task Force (PTF) maintains and refines the Value Pathways powered by the National Comprehensive Cancer Network (NCCN) through systematic review of scientific evidence, emerging literature, treatment toxicity, and cost considerations. The speed with which the PTF reviews US Food and Drug Administration (FDA) approvals and NCCN Guideline updates directly impacts the relevance and utility of pathway recommendations in clinical practice.
Methods
A retrospective analysis was conducted of FDA oncology drug approvals, NCCN Guideline additions, and corresponding PTF discussion dates from January 2024 through June 2025. Metrics included eligibility for PTF discussion, time from FDA approval or guideline update to PTF review, percentage of discussions occurring within 30, 60, and 90 days, and frequency of reviews occurring before official FDA approval or NCCN inclusion.
Results
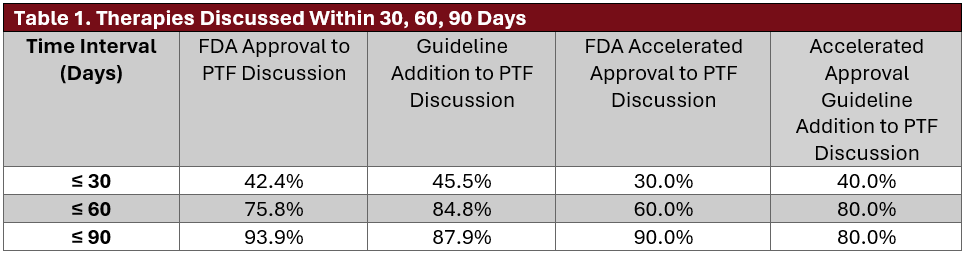
From January 2024 through June 2025, 84 oncology drug approvals were issued by the FDA, of which 34 (40%) were for disease areas addressed by Value Pathways. The average time from FDA approval to PTF discussion was 54 days (median, 49; range, 14–232). The average time from NCCN Guideline inclusion to PTF discussion was also 54 days (median, 37; range, 10–287). Among the 34 therapies, 10 received accelerated approval; the average PTF discussion times for these were 48 days following FDA approval and 49 days following NCCN inclusion. Eight therapies were discussed before FDA approval (mean, 90 days before) and 4 before guideline inclusion (mean, 46 days before). See Table 1 for discussion timelines within 30-, 60-, and 90-day windows.
Conclusion
This evaluation demonstrates the PTF’s responsiveness to newly approved oncology therapies. The 40% eligibility rate reflects the program’s focus on high-impact diseases within community oncology. Most therapies were reviewed within 60 days of FDA or NCCN action, with nearly all discussed within 90 days.
Accelerated approvals showed slightly longer timelines, likely due to the need for more rigorous evidence review. Therapies are initially reviewed by subspecialty subcommittees, which may delay advancement to PTF discussion due to incomplete data, cost concerns, or the need for clarification. Guideline additions may also precede FDA approvals and should be interpreted accordingly.
These findings affirm the PTF’s ability to conduct timely, structured reviews for pathway consideration and offer a benchmark for pathway governance processes in oncology.