
TEVIMBRA® (tislelizumab-jsgr) for Gastric or Gastroesophageal Junction Adenocarcinoma
An interview with Michael K. Gibson, MD, PhD, FACP
Vanderbilt-Ingram Cancer Center, Nashville, TN
This article was developed by HMP with support from BeOne Medicines.
An interview with Michael K. Gibson, MD, PhD, FACP
Vanderbilt-Ingram Cancer Center, Nashville, TN
This article was developed by HMP with support from BeOne Medicines.
Michael K. Gibson, MD, PhD, FACP, discusses the historical management of gastric cancer and introduces a treatment option for gastric or gastroesophageal junction adenocarcinoma.
What unmet needs exist in the treatment of gastric cancer?
Gastric cancer is a multifactorial disease that has both genetic and environmental components.1,2 Globally, gastric cancer is the fifth most diagnosed cancer and the third most common cause of cancer-related death.1 As gastric cancer can be highly aggressive, there is an increased reliance on early detection and prevention strategies. Over the past several decades, there has been a steady decrease in the incidence and mortality rates due to a better understanding of disease progression.1,2
Specific molecular biomarkers, including human epidermal growth factor receptor 2 (HER2), microsatellite instability high (MSI-H), programmed cell death ligand 1 (PD-L1), and claudin 18.2 (CLDN18.2), have been validated as actionable biomarkers in gastric cancer, and biomarker testing can inform personalized treatment strategies.
What treatment options are available for gastric cancer?
Treatment of gastric cancer often uses endoscopic resection, which may be curative in the early stages of the disease. Given the high rate of relapse, neoadjuvant and adjuvant chemotherapy has become standard practice due to the robust data indicating increased rates of survival post-resection.1
Several targeted therapies and immunotherapies that increase patients’ overall survival in the first-line metastatic setting have been approved by the US Food & Drug Administration (FDA), including treatments that target HER2, CLDN18.2, and programmed cell death protein 1 (PD-1).1,3-7
Trastuzumab is indicated in adults, in combination with cisplatin and capecitabine or 5-fluorouracil, for the treatment of patients with HER2 overexpressing metastatic gastric or gastroesophageal junction adenocarcinoma who have not received prior treatment for metastatic disease.3
Zolbetuximab-clzb is a CLDN18.2-directed antibody indicated in combination with fluoropyrimidine- and platinumcontaining chemotherapy for first-line treatment of adults with locally advanced unresectable or metastatic HER2-negative gastric or gastroesophageal junction adenocarcinoma whose tumors are CLDN18.2-positive.4
Nivolumab is a PD-1-blocking antibody indicated in combination with fluoropyrimidine- and platinum-containing chemotherapy for treating adults with advanced or metastatic gastric cancer, gastroesophageal junction cancer, and esophageal adenocarcinoma.5
Pembrolizumab is a PD-1-blocking antibody indicated in combination with trastuzumab, fluoropyrimidine- and platinum-containing chemotherapy for first-line treatment of adults with locally advanced unresectable or metastatic HER2-positive gastric or gastroesophageal junction adenocarcinoma whose tumors express PD-L1 (CPS ≥1); in combination with fluoropyrimidine- and platinum-containing chemotherapy for first-line treatment of adults with locally advanced unresectable or metastatic HER2-negative gastric or gastroesophageal junction adenocarcinoma.6
Tislelizumab-jsgr is a PD-1-blocking antibody indicated in combination with platinum- and fluoropyrimidine-based chemotherapy for the treatment of adult patients with unresectable or metastatic HER2-negative gastric or gastroesophageal junction adenocarcinoma whose tumors express PD-L1 (≥1).7
What is the role of PD-1 in gastric cancer?
PD-1 and its ligands, PD-L1 and PD-L2, maintain immune homeostasis by reducing immune cell targeting to self-tissues via inhibition of T cell activation.8 In the tumor microenvironment, PD-1, expressed on activated T cells, binds to the PDL1 ligand expressed on tumor cells, resulting in T cell apoptosis.9 PD-1 is also expressed by tumor-associated macrophages (TAMs), and overexpression of PD-1 in TAMs results in an increase in tumor growth.10 The binding of PD-1 to its ligands, therefore, exerts an immunosuppressive effect within the tumor microenvironment, disrupting immune homeostasis and allowing tumor cell immune escape.8
What challenges are associated with PD-1 inhibition?
As with many therapeutic targets in oncology, mitigation of resistance to therapy is paramount. Primary resistance to antiPD-1 therapy may result due to insufficient antigen immunogenicity, dysfunction of antigen presentation, irreversible T cell exhaustion, resistance of IFN-γ signaling, and immunosuppressive tumor microenvironment.11 All PD-1 blocking antibodies approved for treating gastric cancer, except tislelizumab-jsgr, mimic the function of wild-type human IgG4, which has an intact Fc region that binds to the Fc gamma receptor on type 1 macrophages, resulting in antibody-dependent cell phagocytosis.12 This process is thought to be a source of resistance to anti–PD-1 therapy.13
Acquired resistance after an initial response to anti–PD-1 therapy is also of concern due to dysfunction in tumor-specific T cells and their inability to develop into memory T cells.11 To overcome resistance, anti–PD-1 antibodies should be paired with additional therapies to enhance T cell priming, reverse T cell exhaustion, increase T cell infiltration, and improve the immunosuppressive microenvironment, thereby increasing the sensitivity of anti–PD-1 therapy.11
What is the design of tislelizumab-jsgr?
Tislelizumab-jsgr is engineered to reduce binding to FcγR on macrophages, helping to prevent antibody-dependent cellular phagocytosis, a process that may lead to anti–PD-1 therapy resistance.12 In preclinical studies, the unique binding orientation of tislelizumab-jsgr to PD-1 resulted in an approximately 30- and 80-fold slower dissociation rate compared with nivolumab and pembrolizumab, respectively. Consequently, tislelizumabjsgr exhibits increased receptor occupancy at lower concentrations.14 Additionally, while tislelizumab-jsgr was shown to block PD-L1 binding completely, other anti–PD-1 therapies produced only partial blocking (~80%) at a concentration of 3 µg/mL in a prelinical model.14
References
- Smyth EC, Nilsson M, Grabsch HI, van Grieken NC, Lordick F. Gastric cancer. Lancet. 2020;396(10251):635-648. doi:10.1016/S0140-6736(20)31288-5
- Machlowska J, Baj J, Sitarz M, Maciejewski R, Sitarz R. Gastric cancer: epidemiology, risk factors, classification, genomic characteristics and treatment strategies. Int J Mol Sci. 2020;21(11):4012. doi:10.3390/ijms21114012
- Herceptin (trastuzumab). Package Insert. Genetech. 2024. South San Francisco, CA.
- Vyloy (zolbetuximab-clzb). Package Insert. Astellas Pharma US, Inc. 2024. Northbrook, IL.
- Opdivo (nivolumab). Package Insert. Bristol Myers Squibb. 2024. Princeton, NJ.
- Keytruda (pembrolizumab). Package Insert. Merck. 2024. Rahway, NJ.
- Tevimbra (tislelizumab-jsgr). Package Insert. BeiGene USA. 2025. San Mateo, CA.
- Lin X, Kang K, Chen P, et al. Regulatory mechanisms of PD-1/PD-L1 in cancers. Mol Cancer. 2024;23(1):108. doi:10.1186/s12943-024-02023-w
- Narita Y, Muro K. Updated immunotherapy for gastric cancer. J Clin Med. 2023;12(7):2636. doi:10.3390/jcm12072636
- Gambardella V, Castillo J, Tarazona N, et al. The role of tumor-associated macrophages in gastric cancer development and their potential as a therapeutic target. Cancer Treat Rev. 2020;86:102015. doi:10.1016/j.ctrv.2020.102015
- Lei Q, Wang D, Sun K, Wang L, Zhang Y. Resistance mechanisms of anti–PD1/PDL1 therapy in solid tumors. Front Cell Dev Biol. 2020;8:672. doi:10.3389/fcell.2020.0067
- Zhang T, Song X, Xu L, et al. The binding of an anti–PD-1 antibody to FcγRΙ has a profound impact on its biological functions. Cancer Immunol Immunother. 2018;67(7):1079-1090. doi:10.1007/s00262-018-2160-x
- Dahan R, Sega E, Engelhardt J, Selby M, Korman AJ, Ravetch JV. FcγRs modulate the anti-tumor activity of antibodies targeting the PD-1/PD-L1 axis [published correction appears in Cancer Cell. 2015;28(4):543]. Cancer Cell. 2015;28(3):285-295. doi:10.1016/j.ccell.2015.08.004
- Hong Y, Feng Y, Sun H, et al. Tislelizumab uniquely binds to the CC’ loop of PD-1 with slow-dissociated rate and complete PD-L1 blockage. FEBS Open Bio. 2021;11(3):782792. doi:10.1002/2211-5463.13102

INDICATION
TEVIMBRA® (tislelizumab-jsgr) is a programmed death receptor-1 (PD-1)-blocking antibody indicated in combination with platinum- and fluoropyrimidine-based chemotherapy for the treatment of adult patients with unresectable or metastatic HER2-negative gastric or gastroesophageal junction adenocarcinoma whose tumors express PD-L1 (≥1).
IMPORTANT SAFETY INFORMATION
WARNINGS AND PRECAUTIONS
Severe and Fatal Immune-Mediated Adverse Reactions
TEVIMBRA is a monoclonal antibody that belongs to a class of drugs that block the PD-1/PD-L1 pathway, thereby removing inhibition of the immune response, potentially breaking peripheral tolerance and inducing immune-mediated adverse reactions. Important immune-mediated adverse reactions listed here may not include all possible severe and fatal immune-mediated reactions.
Immune-mediated adverse reactions, which may be severe or fatal, can occur in any organ system or tissue. Immune-mediated adverse reactions can occur at any time after starting treatment with a PD-1/PD-L1 blocking antibody. While immune-mediated adverse reactions usually manifest during treatment with PD-1/PD-L1 blocking antibodies, immune-mediated adverse reactions can also manifest after discontinuation of PD-1/PD-L1 blocking antibodies. Immune-mediated adverse reactions observed include immune-mediated pneumonitis, immune-mediated colitis, immune-mediated hepatitis, immune-mediated endocrinopathies, immune-mediated nephritis with renal dysfunction, immune-mediated dermatologic adverse reactions, and solid organ transplant rejection.
Please see full Prescribing Information.
PRODUCT INFORMATION
Tislelizumab-jsgr binds to PD-1 and blocks its interaction with PD-L1 and PD-L2, releasing PD-1 pathway-mediated inhibition of the immune response, including the anti-tumor immune response. Tislelizumab-jsgr decreased tumor growth in xenograft models and a human PD-1 transgenic mouse model.
The binding of the PD-1 ligands PD-L1 and PD-L2 to the PD-1 receptor found on T cells inhibits T-cell proliferation and cytokine production. Upregulation of PD-1 ligands occurs in some tumors, and signaling through this pathway can contribute to the inhibition of active T-cell immune surveillance of tumors.
PHARMACODYNAMICS/PHARMACOKINETICS
The tislelizumab-jsgr exposure-response relationship for efficacy and safety and time course of pharmacodynamic response has not been fully characterized.
The peak concentration (Cmax) and area under the plasma concentration versus time curve (AUC) of tislelizumab-jsgr increased dose proportionally in the dose range of 0.5 (0.2 times the approved recommended dosage in a 70 kg patient) to 10 mg/kg (3.5 times the approved recommended dosage in a 70 kg patient). The steady-state AUCtau of tislelizumabjsgr is 1,283 mcg/mL day (28.7%) and the Cmax is 110 mcg/ mL (22.2%) following the approved recommended dosage. Steady-state concentration of tislelizumab-jsgr is reached after 12 weeks of repeated dosing with an every 3-week regimen and the systemic accumulation was 2.14-fold. The tislelizumab-jsgr steady-state total volume of distribution is 6.42 L (32.6%). The tislelizumab-jsgr total clearance is 0.153 L/day (29.5%), and the terminal half-life (t½) is 24 days (31%).
No clinically significant differences in the pharmacokinetics of tislelizumab-jsgr were observed based on age (range, 18 to 90 years), weight (range, 32 to 130 kg), race (White, Asian, or Black), mild to moderate renal impairment (CLcr ≥30 mL/min, estimated by Cockcroft-Gault), mild to moderate hepatic impairment (total bilirubin ≤3 times ULN and any AST, estimated by NCI criteria). The effect of severe hepatic impairment (total bilirubin >3 times ULN and any AST), severe renal impairment (CLcr 15-29 mL/min), or end stage renal disease (CLcr <15 mL/ min) on the pharmacokinetics of tislelizumab-jsgr is unknown.
In patients who received tislelizumab-jsgr in RATIONALE-305 throughout the treatment period and in the ADA analysis set, the incidence of anti-tislelizumab antibodies was 22.7% (108/475). Among the anti-tislelizumab antibody-positive patients, the incidence of neutralizing antibodies was 5.6% (6/108). There was no significant effect of anti-drug antibodies on the pharmacokinetics of tislelizumab-jsgr. The effect of anti-drug antibodies on the pharmacodynamics, safety, or effectiveness of tislelizumab-jsgr has not been fully characterized.
CLINICAL STUDIES IN PATIENTS WITH GASTRIC CANCER
Efficacy
The efficacy of TEVIMBRA in patients with gastric cancer was evaluated in RATIONALE-305, a randomized, multicenter, placebo-controlled, double-blind trial (NCT03777657) in patients with HER2-negative previously untreated unresectable or metastatic gastric or gastroesophageal junction adenocarcinoma. The RATIONALE-305 trial enrolled adults with histologically confirmed, locally advanced unresectable or metastatic gastric or gastroesophageal junction adenocarcinoma and no previous systemic therapy for locally advanced unresectable or metastatic gastric or gastroesophageal junction cancer.
Patients were randomized to receive either TEVIMBRA 200 mg every 3 weeks or placebo in combination with investigator’s choice of chemotherapy on a 21-day cycle. TEVIMBRA (or placebo) was administered until disease progression or unacceptable toxicity. The chemotherapy regimens consisted of:
CAPOX: Oxaliplatin 130 mg/m2 IV on Day 1 for up to 6 cycles and capecitabine 1000 mg/m2 orally twice daily for 14 consecutive days. Capecitabine treatment could be continued beyond 6 cycles, or
FP: Cisplatin 80 mg/m2 IV, Day 1, and 5-FU 800 mg/m2/day IV continuous infusion over 24 hours daily Day 1-5. Cisplatin and 5-FU were given for up to 6 cycles.
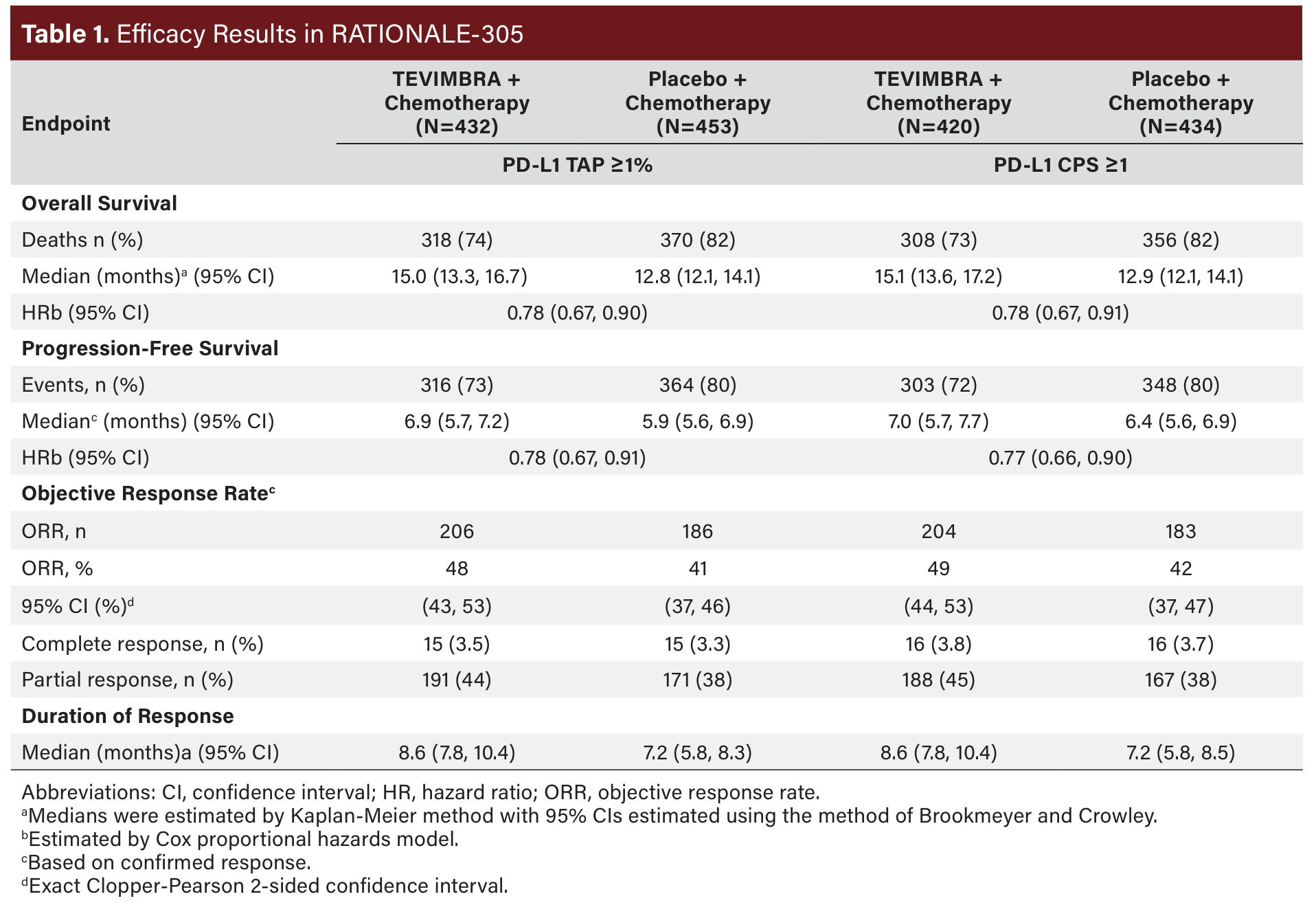
The primary efficacy outcome measures were OS in the PDL1 TAP score ≥5% population and in the Intent-to-Treat (ITT) population. Secondary outcome measures included progressionfree survival (PFS), objective response rate (ORR), and duration of response (DoR) as assessed by the investigator per RECIST v1.1. Additional analyses of efficacy outcome measures were also conducted based on PD-L1 TAP ≥1% and CPS ≥1.
A total of 997 patients were randomized. The trial population characteristics were median age 61 years (range, 23 to 86 years), 35% ≥65 years of age, 69% male; 75% Asian, 22% White, and 0% Black or African American. Eighty percent had primary stomach tumor; 89% had PD-L1 TAP ≥1% and 86% had PD-L1 CPS ≥1, and 99% of patients had metastatic disease at baseline. Baseline ECOG performance status was 0 (32%) or 1 (68%). Ninety-three percent of patients received CAPOX and 7% received FP.
RATIONALE-305 demonstrated a statistically significant improvement in OS for patients randomized to TEVIMBRA in combination with chemotherapy compared with placebo plus chemotherapy in the PD-L1 TAP ≥5% population and in the ITT population. Exploratory analyses of OS in the TAP <1% population and in the CPS <1 population showed hazard ratios of 0.98 (95% CI: 0.64, 1.50) and 1.01 (95% CI: 0.66, 1.52) respectively, indicating that the improvement in the ITT population was primarily attributed to the results observed in the subgroup of patients with PD-L1 ≥1 (Table 1). An exploratory subgroup analysis of OS in 40 patients with MSI-H tumors irrespective of PD-L1 status showed a HR of 0.66 (0.3, 1.43).
Safety
The safety of TEVIMBRA in combination with chemotherapy was evaluated in RATIONALE-305. Serious adverse reactions occurred in 42% of patients receiving TEVIMBRA in combination with chemotherapy. The most frequent serious adverse drug reactions (≥2%) were pneumonia (3.6%), decreased platelet count (3.2%), gastrointestinal hemorrhage (3%), and colitis (2.2%). Fatal adverse reactions occurred in 4.2% of patients who received TEVIMBRA in combination with chemotherapy; events occurring in 2 or more patients were death, sepsis, pneumonia, pulmonary embolism, and respiratory failure.
Permanent discontinuation of TEVIMBRA due to an adverse reaction occurred in 16% of patients. Adverse drug reactions which resulted in permanent discontinuation in ≥1% of patients were death, fatigue, and pneumonitis.
Dosage interruption of TEVIMBRA due to an adverse drug reaction occurred in 49% of patients. Adverse drug reactions which required dosage interruption in ≥2% of patients were decreased platelet count (12%), decreased neutrophil count (10%), neutropenia (6%), decreased white blood cell count (6%), increased AST (4.8%), increased ALT (3.8%), increased blood bilirubin (3%), COVID-19 (3%), thrombocytopenia (2.8%), leukopenia (2.6%), pneumonitis (2.2%), and pneumonia (2%).
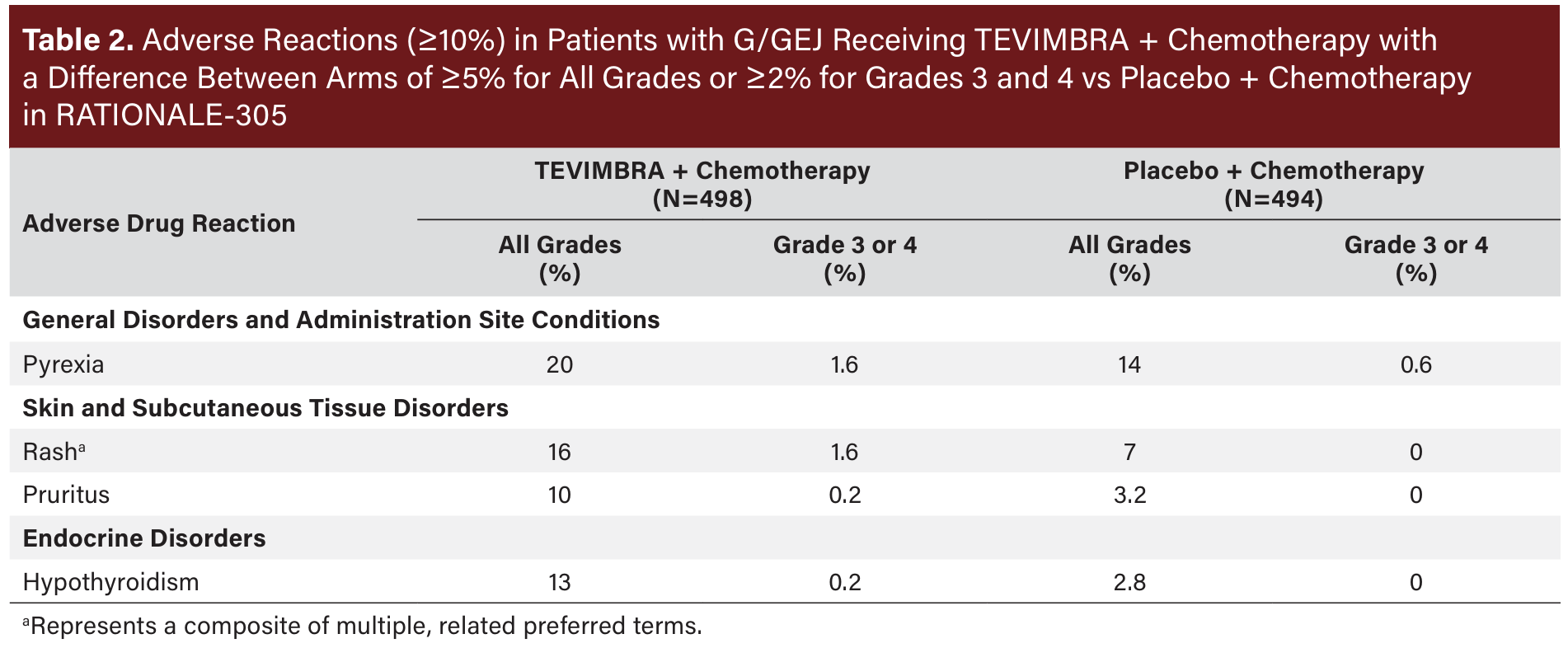
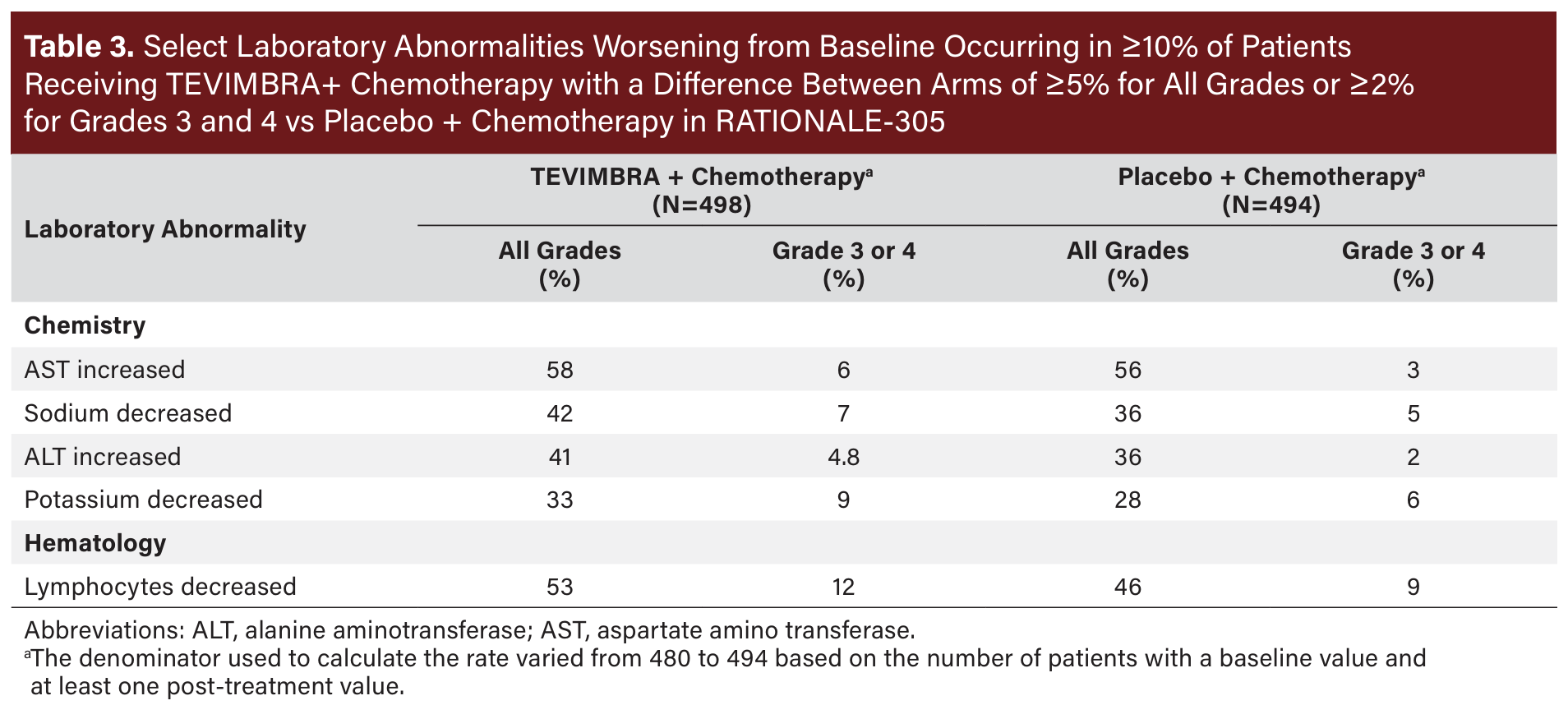
The most common (≥20%) adverse reactions, including laboratory abnormalities, for TEVIMBRA in combination with chemotherapy were nausea, fatigue, decreased appetite, anemia, peripheral sensory neuropathy, vomiting, decreased platelet count, decreased neutrophil count, increased aspartate aminotransferase, diarrhea, abdominal pain, increased alanine aminotransferase, white blood cell count decreased, decreased weight, and pyrexia. See Table 2 for additional data on adverse reactions and Table 3 for laboratory abnormality data from RATIONALE-305.
DOSAGE AND ADMINISTRATION
TEVIMBRA is available as a 100 mg/10 mL solution in a single-dose vial. TEVIMBRA should be stored in a refrigerator at 2°C to 8°C (36°F to 46°F) in the original carton to protect from light. The recommended dosage of TEVIMBRA administered intravenously as a single agent or in combination with platinum and fluoropyrimidine-containing chemotherapy is 150 mg every 2 weeks or 200 mg every 3 weeks or 300 mg every 4 weeks until disease progression or unacceptable toxicity.
Prepare the solution for infusion as follows:
- Withdraw the required volume of TEVIMBRA from the vial(s).
- Transfer solution into an intravenous infusion bag containing 0.9% Sodium Chloride Injection, USP to prepare an infusion solution with a final concentration of 2 mg/mL to 5 mg/mL.
- Mix diluted solution by gentle inversion to avoid foaming or excessive shearing of the solution. Do not shake.
- TEVIMBRA is for single use only. Discard any unused portion left in the vial.
As TEVIMBRA does not contain any preservatives, if not used immediately, store the TEVIMBRA diluted solution either:
- At room temperature at 20°C to 25°C (68°F to 77°F) for no more than 4 hours, including preparation and infusion duration. Discard after 4 hours.
- Under refrigeration at 2°C to 8°C (36°F to 46°F) for up to 20 hours, including preparation and infusion duration. Allow the diluted solution to come to room temperature prior to administration. Discard after 20 hours.
Do not freeze the diluted solution.
No dose reduction of TEVIMBRA is recommended. In general, withhold TEVIMBRA for severe (Grade 3) immune-mediated adverse reactions. Permanently discontinue TEVIMBRA for life-threatening (Grade 4) immune-mediated adverse reactions, recurrent severe (Grade 3) immune-mediated reactions that require systemic immunosuppressive treatment, or an inability to reduce corticosteroid dose to 10 mg or less of prednisone equivalent per day within 12 weeks of initiating steroids.
SUMMARY
Tislelizumab-jsgr is a unique anti–PD-1 antibody that demonstrates improved PD-1 binding characteristics compared with previously approved anti–PD-1 antibodies in gastric cancer. In patients with previously untreated unresectable or metastatic gastric or gastroesophageal junction cancer, tislelizumab-jsgr in combination with chemotherapy demonstrated a clinically meaningful improvement compared with placebo in combination with chemotherapy in overall survival, progression-free survival, objective response rate, and duration of response. This indicates that tislelizumab-jsgr may be an effective addition to the treatment arsenal for patients with gastric or gastroesophageal junction cancer whose tumors express PD-L1 (≥1).













