
TEVIMBRA® (tislelizumab-jsgr) for Esophageal Squamous Cell Carcinoma
An interview with Jaffer A. Ajani, MD
The University of Texas MD Anderson Cancer Center, Houston, TX
This article was developed by HMP with support from BeOne Medicines.
An interview with Jaffer A. Ajani, MD
The University of Texas MD Anderson Cancer Center, Houston, TX
This article was developed by HMP with support from BeOne Medicines.
Jaffer A. Ajani, MD, reviews the available treatment options for esophageal cancer, including a unique compound that is designed to more effectively block the PD-1 receptor.
What unmet needs exist in the treatment of ESCC?
Globally, esophageal cancer (EC) is the ninth most diagnosed cancer and the sixth most common cause of cancer-related death.1 There are two primary subtypes of EC, each with distinct clinical features: esophageal squamous cell carcinoma (ESCC) and esophageal adenocarcinoma (EAC).1 ESCC is a multifactorial disease, with risks that include lifestyle factors, such as tobacco and alcohol use, low socioeconomic status, poor nutrition, and poor oral hygiene.1
Compared to EAC, ESCC more frequently expresses programmed cell death ligand 1 (PD-L1) protein.2 To date, PD-L1 expression for programmed cell death protein 1 (PD-1) immunotherapy is the only clinically applicable biomarker for first-line treatment of ESCC.3
What treatment options are available for ESCC?
In patients with pre-malignant lesions and early-stage ESCC, endoscopic eradication therapy is often the best treatment strategy. Removal via endoscopic mucosal resection or endoscopic submucosal dissection is an additional treatment strategy, depending on the available endoscopic expertise.1 In more advanced ESCC, curative treatment typically includes chemotherapy or chemoradiotherapy paired with esophagectomy, though long-term outcomes remain poor.1
Immunotherapy treatments that target PD-1 and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) to treat ESCC have increased since FDA approval.4-7 The National Comprehensive Cancer Network (NCCN) Guidelines® recommend the following immunotherapy combinations for first-line treatment of ESCC: fluoropyrimidine, oxaliplatin/cisplatin, and nivolumab for PD-L1 combined positive score (CPS) ≥1 (category 1); fluoropyrimidine, oxaliplatin/cisplatin, and pembrolizumab for PD-L1 CPS ≥1 (category 1); fluoropyrimidine, oxaliplatin/cisplatin, and tislelizumab-jsgr for PD-L1 CPS ≥1 (category 1); oxaliplatin/cisplatin, paclitaxel, and tislelizumab-jsgr for PD-L1 CPS ≥1; and nivolumab and ipilimumab for PD-L1 CPS ≥1.8 For tumors with high micro-satellite instability or mismatch repair deficiency independent of PD-L1 status, the preferred regimens include pembrolizumab, dostarlimab-gxly, nivolumab and ipilimumab, or the combination of fluoropyrimidine and oxaliplatin with either nivolumab or pembrolizumab.8 NCCN Guidelines for second-line or subsequent treatment of ESCC with immunotherapy independent of PD-L1 status include monotherapy with either nivolumab (category 1) or tislelizumab-jsgr (category 1). The use of pembrolizumab monotherapy as a second-line ESCC treatment requires PD-L1 CPS ≥10 (category 1).8
Nivolumab is a PD-1 blocking antibody indicated in adults with completely resected EC with residual pathologic disease, who have received neoadjuvant chemoradiotherapy.4 Furthermore, nivolumab is indicated in combination with fluoropyrimidine- and platinum-containing chemotherapy as first-line treatment for adults with unresectable advanced or metastatic ESCC tumors that express PD-L1 (≥1).4 Nivolumab may also be used in combination with ipilimumab as first-line therapy in adults with unresectable advanced or metastatic ESCC tumors that express PD-L1 (≥1).4 Finally, nivolumab is indicated as a single agent in adults with unresectable advanced, recurrent, or metastatic ESCC after prior fluoropyrimidine- and platinum-based chemotherapy.4
Ipilimumab is a CTLA-4-blocking antibody indicated in combination with nivolumab as a first-line treatment for adults with unresectable advanced or metastatic ESCC.5
Pembrolizumab is a PD-1 blocking antibody indicated in combination with platinum- and fluoropyrimidine-based chemotherapy as first-line treatment for adults with locally advanced or metastatic EC tumors that express PD-L1 (CPS ≥1) and are not amenable to surgical resection or definitive chemoradiation, or as a single agent after ≥1 prior line of systemic therapy for ESCC tumors that express PD-L1 (CPS ≥10) as determined by an FDA-approved test.6
Tislelizumab-jsgr is a PD-1 blocking antibody indicated in combination with platinum-containing chemotherapy for the first-line treatment of adults with unresectable or metastatic ESCC whose tumor expresses PD-L1 (≥1).7 Tislelizumab-jsgr is also indicated as a single agent in adults with unresectable or metastatic ESCC after prior systemic chemotherapy that did not include a PD-L1 inhibitor.7
What is the role of PD-1 in ESCC?
The role of PD-1 and its ligands, PD-L1 and PD-L2, is to maintain immune homeostasis via the inhibition of activated T cells.9 PD-1, expressed on activated T cells, binds to the PD-L1 ligand expressed on tumor cells or immune cells in the tumor microenvironment (TME), resulting in T cell exhaustion or apoptosis.9 PD-L1 is also expressed by tumor-associated macrophages (TAMs), and upregulation of PD-L1 in TAMs by tumor cells suppresses T cell proliferation and promotes tumor growth.9 In parallel, TAMs upregulate PD-L1 expression in tumor cells, which increases PD-1 expression in TAMs, thereby promoting macrophage polarization to the M2 phenotype.9 The binding of PD-L1 promotes inflammation within the TME, disrupting immune homeostasis and allowing tumor cell immune escape.9
PD-L1 is frequently expressed by ESCC cells or immune cells in the TME, and over 82% of patients with ESCC have PD-L1 expression.10 The high expression of PD-L1 in patients with ESCC is associated with poorer prognosis, higher postoperative recurrence rate, and lower survival rate.11
What challenges are associated with PD-1 inhibition?
As with many therapeutic targets in oncology, mitigation of resistance to therapy is paramount. Primary resistance to anti–PD-1 therapy may result from insufficient antigen immunogenicity, dysfunctional antigen presentation, irreversible T cell exhaustion, resistance to interferon (IFN)-γ signaling, and an immunosuppressive TME.12 All PD-1 blocking antibodies approved for treating ESCC, except tislelizumab-jsgr, mimic the function of wild-type human IgG4, which has an intact Fc region that binds to the Fc gamma receptor on type 1 macrophages, resulting in antibody-dependent cell phagocytosis.13 This process is thought to be a source of resistance to anti–PD-1 therapy.14
Acquired resistance after an initial response to anti–PD-1 therapy is also of concern due to dysfunction in tumor-specific T cells and their inability to develop into memory T cells.12 To overcome resistance, anti–PD-1 antibodies may be paired with additional therapies to enhance T cell priming, reverse T cell exhaustion, increase T cell infiltration, and improve the immunosuppressive TME, thereby increasing the sensitivity of anti–PD-1 therapy.12
The use of biomarkers and identification of patient-specific immunogenic features to optimize the efficacy of anti–PD-1 therapy remains less than optimal. For example, data suggest that patients with NOTCH1 mutations may not respond as favorably to nivolumab plus ipilimumab treatment. At the same time, lower stromal gene expression signatures were associated with an increased survival benefit.3 A recent study of patients receiving tislelizumab-jsgr as second-line treatment also observed that NOTCH1 mutations correlated with a reduction in NOTCH1 signaling in patients with ESCC. Thus, patients with ESCC and NOTCH1 mutations may possess microenvironments with increased IFN-I gene signatures and decreased immune-suppressive cell infiltration.15
A recent decision by the FDA Oncologic Drugs Advisory Committee recommended a class-level cut-off of anti–PD-1 therapies for patients with 1L ESCC who have a PD-L1 expression ≥1.16 While data suggest anti–PD-1 therapies are efficacious as second-line therapy in ESCC,17 the impact this decision will have on the current treatment paradigm remains to be seen.
What makes tislelizumab-jsgr a unique compound?
Tislelizumab-jsgr is engineered to reduce binding to FcγR on macrophages, helping to prevent antibody-dependent cellular phagocytosis, a process that may lead to anti–PD-1 therapy resistance.13 In preclinical studies, the unique binding orientation of tislelizumab-jsgr to PD-1 produced higher affinity than other anti–PD-1 drugs, resulting in an approximately 30- and 80-fold slower dissociation rate from the PD-1 receptor compared with nivolumab and pembrolizumab, respectively. Tislelizumab-jsgr also occupies a larger surface area of the PD-1 receptor, not only preventing binding of PD-L1, but also PD-L2.18 Consequently, tislelizumab-jsgr is more efficient in blocking the PD-1 receptor at lower concentrations, a unique preclinical advantage.18
References
- Sheikh M, Roshandel G, McCormack V, Malekzadeh R. Current status and suture prospects for esophageal cancer. Cancers (Basel). 2023;15(3):765. doi:10.3390/cancers15030765
- Huang TX, Fu L. The immune landscape of esophageal cancer. Cancer Commun (Lond). 2019;39(1):79. doi:10.1186/s40880-019-0427-z
- Jubashi A, Kotani D, Kojima T, Takebe N, Shitara K. Current landscape of targeted therapy in esophageal squamous cell carcinoma. Curr Probl Cancer. 2024;53:101152. doi:10.1016/j.currproblcancer.2024.101152
- Opdivo (nivolumab). Package Insert. Bristol Myers Squibb. 2025. Princeton, NJ.
- Yervoy (ipilimumab). Package Insert. Bristol Myers Squibb. 2025. Princeton, NJ.
- Keytruda (pembrolizumab). Package Insert. Merck. 2025. Rahway, NJ.
- Tevimbra (tislelizumab-jsgr) Package Insert. BeiGene. 2025. San Mateo, CA.
- National Comprehensive Cancer Network. Esophageal and Esophagogastric Junction Cancers. (Version 3.2025). https://www.nccn.org/professionals/physician_gls/pdf/esophageal.pdf. Accessed March 19, 2025.
- Lin X, Kang K, Chen P, et al. Regulatory mechanisms of PD-1/PD-L1 in cancers. Mol Cancer. 2024;23(1):108. doi:10.1186/s12943-024-02023-w
- US Food and Drug Administration. Immune checkpoint inhibitors in patients with metastatic or unresectable esophageal squamous cell carcinoma. Oncologic Drugs Advisory Committee Meeting. September 2024. https://www.fda.gov/media/182143/download.
- Li Q, Liu T, Ding Z. Neoadjuvant immunotherapy for resectable esophageal cancer: a review. Front Immunol. 2022;13:1051841. doi:10.3389/fimmu.2022.1051841
- Lei Q, Wang D, Sun K, Wang L, Zhang Y. Resistance mechanisms of anti–PD1/PDL1 therapy in solid tumors. Front Cell Dev Biol. 2020;8:672. doi:10.3389/fcell.2020.00672
- Zhang T, Song X, Xu L, et al. The binding of an anti–PD-1 antibody to FcγRΙ has a profound impact on its biological functions. Cancer Immunol Immunother. 2018;67(7):1079-1090. doi:10.1007/s00262-018-2160-x
- Dahan R, Sega E, Engelhardt J, Selby M, Korman AJ, Ravetch JV. FcγRs modulate the anti-tumor activity of antibodies targeting the PD-1/PD-L1 axis [published correction appears in Cancer Cell. 2015;28(4):543]. Cancer Cell. 2015;28(3):285-295. doi:10.1016/j.ccell.2015.08.004
- Lu Z, Du W, Jiao X, et al. NOTCH1 mutation and survival analysis of tislelizumab in advanced or metastatic esophageal squamous cell carcinoma: a biomarker analysis from the randomized, phase III, RATIONALE-302 trial. J Clin Oncol. Published online ahead of print April 3, 2025. doi:10.1200/JCO-24-01818
- September 26, 2024 Meeting of the Oncologic Drugs Advisory Committee (ODAC). YouTube. September 26, 2024. Accessed April 15, 2025. https://www.youtube.com/live/ELA3JDqtcFw.
- Zhu X, Shanzhou Q, Li D, Pang X, Ma D. PD-1 inhibitors versus chemotherapy as second-line treatment for advanced esophageal squamous cell carcinoma: a meta-analysis. BMC Cancer. 2021;21(1):1195. doi:10.1186/s12885-021-08958-3
- Hong Y, Feng Y, Sun H, et al. Tislelizumab uniquely binds to the CC' loop of PD-1 with slow-dissociated rate and complete PD-L1 blockage. FEBS Open Bio. 2021;11(3):782-792. doi:10.1002/2211-5463.13102

INDICATION
TEVIMBRA® (tislelizumab-jsgr) is a programmed death receptor-1 (PD-1)-blocking antibody indicated in combination with platinum- and fluoropyrimidine-based chemotherapy for the treatment of adult patients with unresectable or metastatic HER2-negative gastric or gastroesophageal junction adenocarcinoma whose tumors express PD-L1 (≥1).
IMPORTANT SAFETY INFORMATION
WARNINGS AND PRECAUTIONS
Severe and Fatal Immune-Mediated Adverse Reactions
TEVIMBRA is a monoclonal antibody that belongs to a class of drugs that block the PD-1/PD-L1 pathway, thereby removing inhibition of the immune response, potentially breaking peripheral tolerance and inducing immune-mediated adverse reactions. Important immune-mediated adverse reactions listed here may not include all possible severe and fatal immune-mediated reactions.
Immune-mediated adverse reactions, which may be severe or fatal, can occur in any organ system or tissue. Immune-mediated adverse reactions can occur at any time after starting treatment with a PD-1/PD-L1 blocking antibody. While immune-mediated adverse reactions usually manifest during treatment with PD-1/PD-L1 blocking antibodies, immune-mediated adverse reactions can also manifest after discontinuation of PD-1/PD-L1 blocking antibodies. Immune-mediated adverse reactions observed include immune-mediated pneumonitis, immune-mediated colitis, immune-mediated hepatitis, immune-mediated endocrinopathies, immune-mediated nephritis with renal dysfunction, immune-mediated dermatologic adverse reactions, and solid organ transplant rejection.
Please see full Prescribing Information.
PRODUCT INFORMATION
Tislelizumab-jsgr binds to PD-1 and blocks its interaction with PD-L1 and PD-L2, releasing PD-1 pathway-mediated inhibition of the immune response, including the anti-tumor immune response. Tislelizumab-jsgr decreased tumor growth in xenograft models and a human PD-1 transgenic mouse model.
The binding of the PD-1 ligands PD-L1 and PD-L2 to the PD-1 receptor found on T cells inhibits T-cell proliferation and cytokine production. Upregulation of PD-1 ligands occurs in some tumors, and signaling through this pathway can contribute to the inhibition of active T-cell immune surveillance of tumors.
PHARMACODYNAMICS/PHARMACOKINETICS
The tislelizumab-jsgr exposure-response relationship for efficacy and safety and time course of pharmacodynamic response has not been fully characterized.
The peak concentration (Cmax) and area under the plasma concentration versus time curve (AUC) of tislelizumab-jsgr increased dose proportionally in the dose range of 0.5 (0.2 times the approved recommended dosage in a 70 kg patient) to 10 mg/kg (3.5 times the approved recommended dosage in a 70 kg patient). The steady-state AUCtau of tislelizumabjsgr is 1,283 mcg/mL day (28.7%) and the Cmax is 110 mcg/ mL (22.2%) following the approved recommended dosage. Steady-state concentration of tislelizumab-jsgr is reached after 12 weeks of repeated dosing with an every 3-week regimen and the systemic accumulation was 2.14-fold. The tislelizumab-jsgr steady-state total volume of distribution is 6.42 L (32.6%). The tislelizumab-jsgr total clearance is 0.153 L/day (29.5%), and the terminal half-life (t½) is 24 days (31%).
No clinically significant differences in the pharmacokinetics of tislelizumab-jsgr were observed based on age (range, 18 to 90 years), weight (range, 32 to 130 kg), race (White, Asian, or Black), mild to moderate renal impairment (CLcr ≥30 mL/min, estimated by Cockcroft-Gault), mild to moderate hepatic impairment (total bilirubin ≤3 times ULN and any AST, estimated by NCI criteria). The effect of severe hepatic impairment (total bilirubin >3 times ULN and any AST), severe renal impairment (CLcr 15-29 mL/min), or end stage renal disease (CLcr <15 mL/ min) on the pharmacokinetics of tislelizumab-jsgr is unknown.
In patients who received tislelizumab-jsgr in RATIONALE-305 throughout the treatment period and in the ADA analysis set, the incidence of anti-tislelizumab antibodies was 22.7% (108/475). Among the anti-tislelizumab antibody-positive patients, the incidence of neutralizing antibodies was 5.6% (6/108). There was no significant effect of anti-drug antibodies on the pharmacokinetics of tislelizumab-jsgr. The effect of anti-drug antibodies on the pharmacodynamics, safety, or effectiveness of tislelizumab-jsgr has not been fully characterized.
CLINICAL STUDIES IN PATIENTS WITH ESCC
Efficacy in First-line ESCC Patients
The efficacy of tislelizumab-jsgr as first-line treatment in patients with ESCC was evaluated in RATIONALE-306 (NCT03783442), a global, randomized, placebo-controlled, double-blind study in patients with unresectable, recurrent, or metastatic esophageal squamous cell carcinoma (ESCC).
Patients were enrolled regardless of their PD-L1 expression level. PD-L1 expression was evaluated at a central laboratory using the Ventana PD-L1 (SP263) assay that identified PD-L1 staining on both tumor and tumor-associated immune cells (Tumor Area Positivity or TAP). A retrospective scoring of tumor PD-L1 status using Combined Positive Score (CPS) was also conducted using the PD-L1–stained tumor specimens used for randomization.
Patients should not have received prior systemic therapy for advanced or metastatic disease. A treatment-free interval of at least 6 months was required if there was prior neoadjuvant/adjuvant therapy with platinum-based chemotherapy. The trial excluded patients who had active leptomeningeal disease or uncontrolled brain metastasis, active autoimmune disease, a medical condition requiring systemic corticosteroids or immunosuppressants, or evidence of fistula or complete esophageal obstruction not amenable to treatment.
Patients were randomized (1:1) to receive either TEVIMBRA 200 mg every 3 weeks or placebo in combination with investigator’s choice of chemotherapy (ICC) on a 21-day cycle. Patients received TEVIMBRA until disease progression assessed by the investigator per RECIST v1.1, or until unacceptable toxicity. The chemotherapy doublet regimen consists of:
- Platinum (cisplatin [60 to 80 mg/m2 IV, on Day 1] or oxaliplatin [130 mg/m2 IV, on Day 1]) and a fluoropyrimidine (fluorouracil [750 to 800 mg/m2 IV, on Days 1 to 5] or capecitabine [1000 mg/m2 orally twice daily, on Days 1 to 14]),
or
- Platinum (cisplatin [60 to 80 mg/m2 IV, on Day 1 or 2] or oxaliplatin [130 mg/m2 IV, on Day 1 or 2]) and (paclitaxel 175 mg/m2 IV, on Day 1)
A total of 649 patients were randomized. The trial population characteristics were median age 64 years (range: 26 to 84 years), 48% were ≥65 years of age, 87% were male, 75% were Asian, and 24% were White. Eighty-six percent had metastatic disease and 14% had locally advanced disease; 99.8% of patients had histological confirmation of squamous cell carcinoma. Baseline ECOG performance status was 0 (33%) or 1 (67%). Thirty-four percent of patients had tumors that expressed PDL1 TAP ≥10%, 74% had PD-L1 TAP ≥1%, and 74% had PD-L1 CPS ≥1. Fifty-five percent of patients received platinum (cisplatin or oxaliplatin) and paclitaxel-containing regimens, and 45% received platinum (cisplatin or oxaliplatin) and fluoropyrimidine-containing regimens.
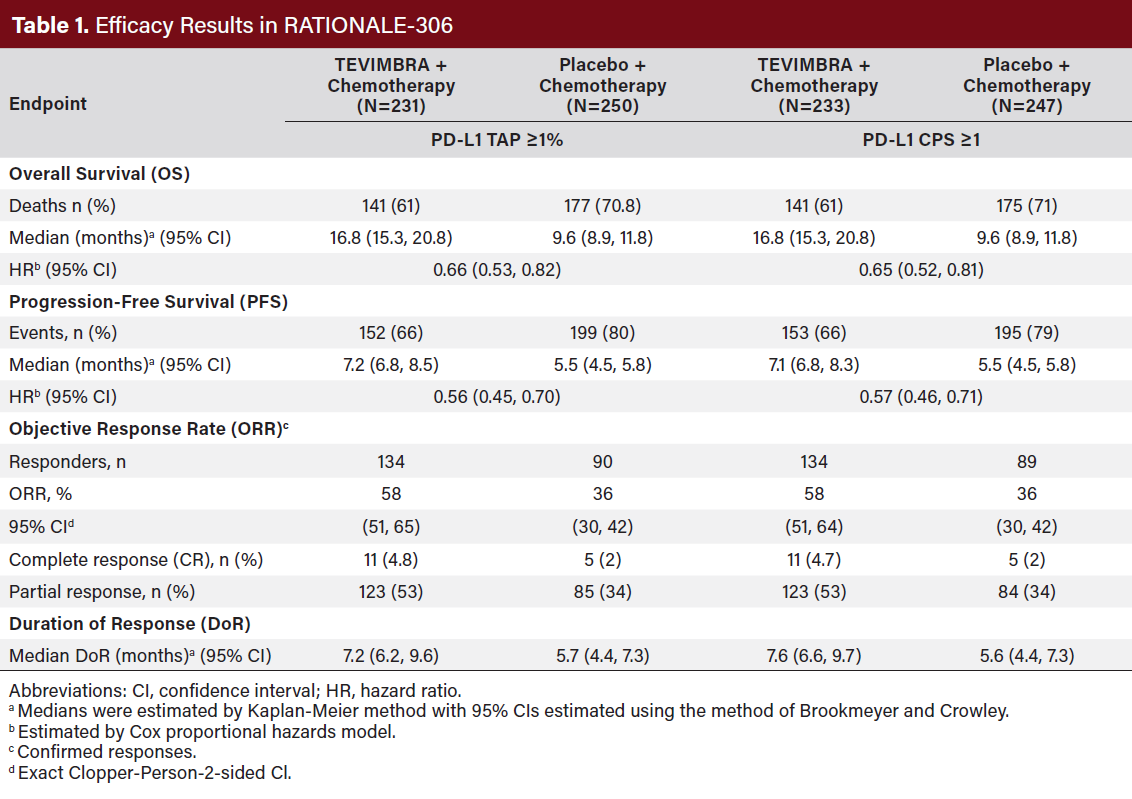
RATIONALE-306 demonstrated a statistically significant improvement in OS for patients randomized to TEVIMBRA in
combination with chemotherapy compared to placebo in combination with chemotherapy. Exploratory analysis of OS in the population with TAP <1% population and in the CPS <1 population showed hazard ratios of 1.34 (95% CI 0.73, 2.46) and 1.52(95% CI 0.81, 2.84), respectively, indicating that the improvement in the ITT population was primarily attributed to the results observed in the subgroup of patients with PD-L1 ≥1 (Table 1).
Safety in First-line ESCC Patients
The safety of TEVIMBRA in combination with chemotherapy was evaluated in RATIONALE-306, a randomized, placebo-controlled, multicenter, double-blind trial in patients with unresectable, advanced, or metastatic ESCC. Patients were treated until disease progression or unacceptable toxicity. The median duration of exposure was 6.4 months (range: 0.1 to 38.3 months) in TEVIMBRA-treated patients. Serious adverse reactions occurred in 48% of patients receiving TEVIMBRA in combination with chemotherapy. The most frequent serious adverse reactions (≥2%) were pneumonia (5.2%), dysphagia (5.2%), diarrhea (2.2%), fatigue (2.2%), and esophageal stenosis (2.2%). Fatal adverse reactions occurred in 8% of patients who received TEVIMBRA in combination with chemotherapy.
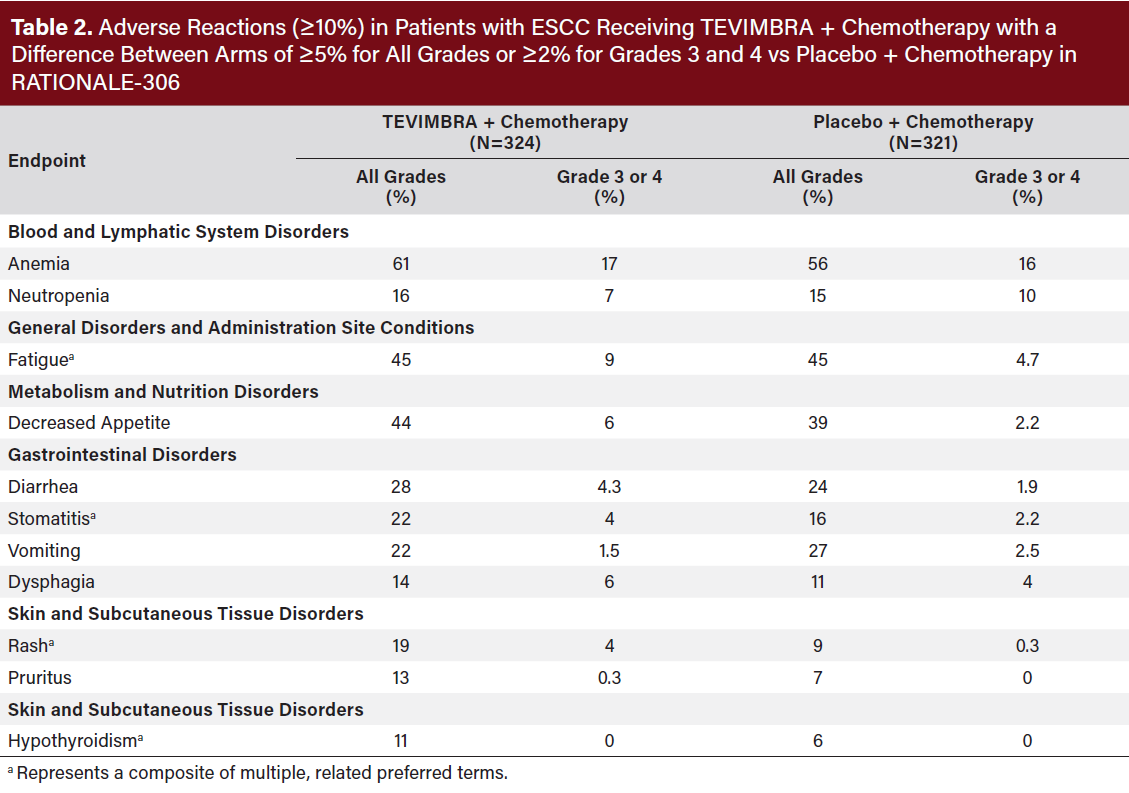
Permanent discontinuation of TEVIMBRA due to adverse reactions occurred in 13% of patients. The adverse reaction which resulted in discontinuation in ≥2% of patients was pneumonitis (2.2%).
Dosage interruptions of TEVIMBRA due to adverse reactions occurred in 52% of patients. Adverse reactions which required dosage interruption in ≥2% of patients were neutrophil count decreased (7%), fatigue (6%), pneumonia (6%),
anemia (4.3%), neutropenia (4.3%), white blood cell count decreased (4.3%), rash (3.7%), dysphagia (2.8%), platelet count decreased (2.8%), pyrexia (2.8%), and diarrhea (2.2%).
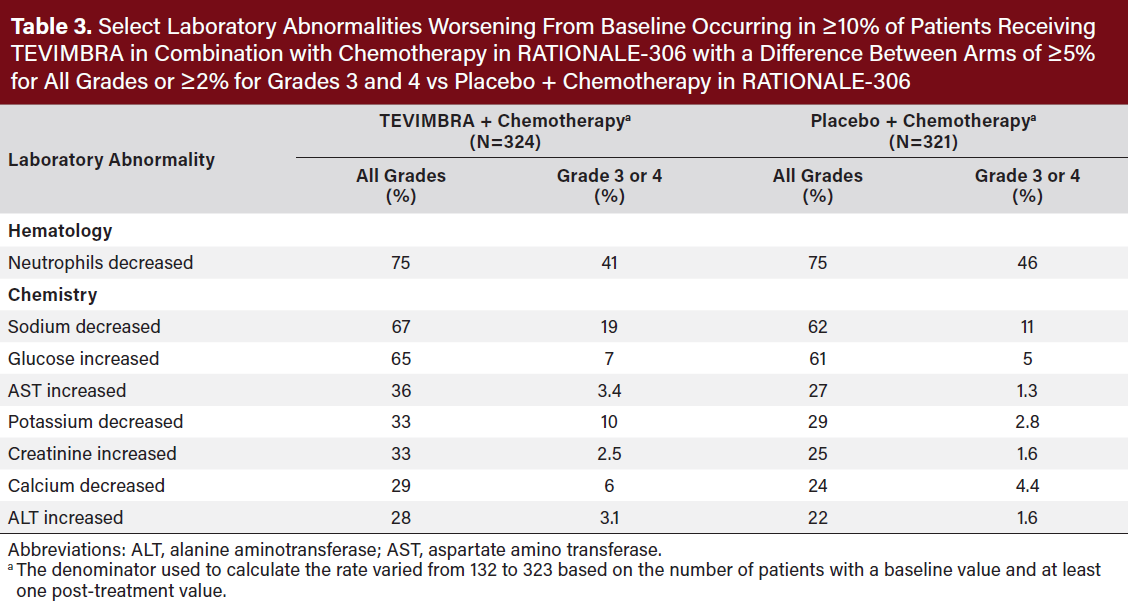
The most common (≥20%) adverse reactions including laboratory abnormalities were decreased neutrophil count, decreased sodium, increased glucose, anemia, fatigue, decreased appetite, increased AST, decreased potassium, increased serum creatinine, decreased calcium, increased ALT, diarrhea, stomatitis, and vomiting (Table 2,3).
Efficacy in Second-line ESCC Patients
The efficacy of TEVIMBRA as second-line treatment in patientswith ESCC was assessed in RATIONALE-302, a randomized, multicenter, placebo-controlled, double-blind trial (NCT03430843) in patients unresectable advanced or metastatic ESCC who progressed on or after prior systemic chemotherapy. The RATIONALE-302 trial enrolled 512 adults with histologically confirmed diagnosis of ESCC, tumor progression during or after first-line treatment for advanced unresectable or metastatic ESCC, at least one measurable/evaluable lesion by Response Evaluation Criteria in Solid Tumors (RECIST) v1.1, and Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1 prior to randomization.
Patients were randomized (1:1) to receive either TEVIMBRA 200 mg every 3 weeks or investigator’s choice of chemotherapy (ICC), all given intravenously: paclitaxel 135-175 mg/m2 every 3 weeks or 80 to 100 mg/m2 weekly, docetaxel 75 mg/m2 every 3 weeks, or irinotecan 125 mg/m2 on Days 1 and 8 of every 3-week cycle. Patients were treated until disease progression assessed by the investigator or unacceptable toxicity.
Randomization was stratified by geographic region (Asia [excluding Japan] vs Japan vs US/EU), ECOG performance
status (0 vs 1), and ICC option. Tumor assessments were conducted every 6 weeks for the first 6 months, then every 9 weeks until disease progression.
The major efficacy outcome measure was overall survival (OS) in the Intent-to-Treat (ITT) population. Additional efficacy outcome measures were investigator-assessed progression-free survival (PFS), overall response rate (ORR), and duration of response (DOR) per RECIST v1.1.
A total of 512 patients were enrolled and randomized to TEVIMBRA (n=256) or ICC (n=256) (irinotecan [46%], paclitaxel [33%], or docetaxel [21%]). Of the 512 patients, 142 (28%) had PD-L1 ≥10%, 222 (43%) had PD-L1 <10%, and 148 (29%) had unknown baseline PD-L1 status. The trial population characteristics were: median age of 62 years (range: 35 to 86), 38% age ≥65; 84% male; 19% White and 80% Asian; 95% had metastatic disease. All patients had received at least one prior anti-cancer systemic therapy. Baseline ECOG performance status was 0 (25%) or 1 (75%).
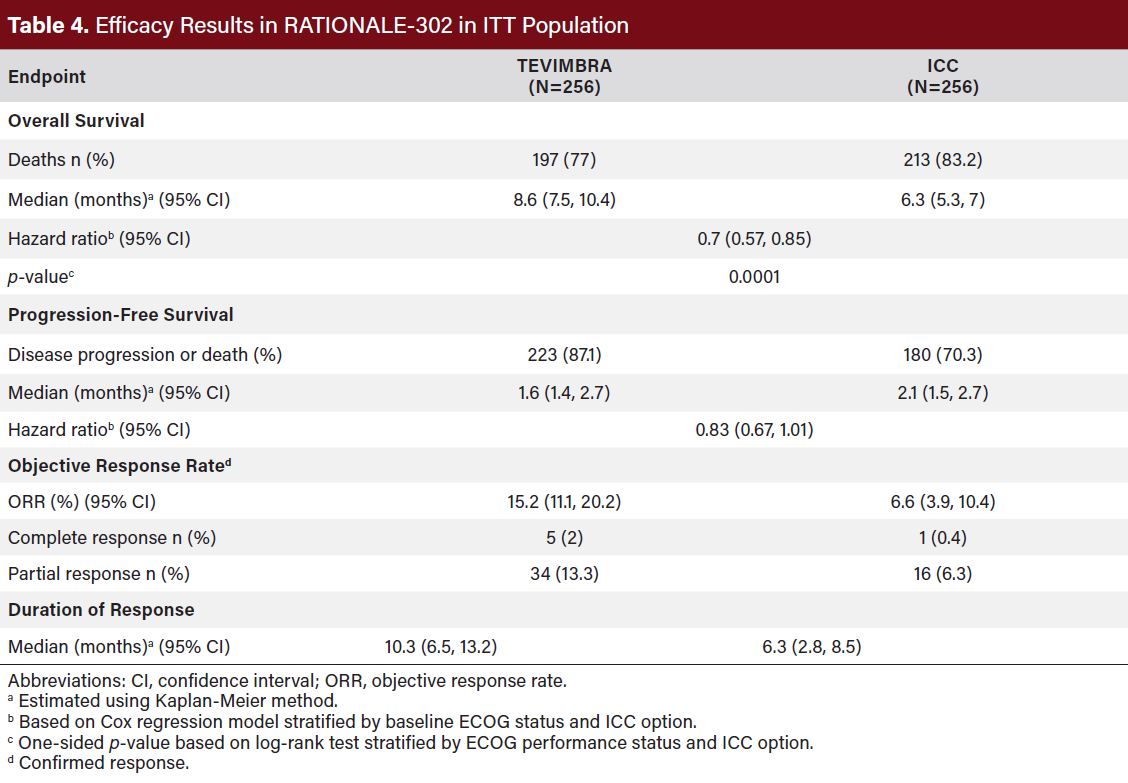 RATIONALE-302 demonstrated a statistically significant improvement in OS for patients randomized to TEVIMBRA as compared with ICC. OS results by PD-L1 CPS level (<1 and ≥1) were not studied (Table 4).
RATIONALE-302 demonstrated a statistically significant improvement in OS for patients randomized to TEVIMBRA as compared with ICC. OS results by PD-L1 CPS level (<1 and ≥1) were not studied (Table 4).
Safety in Second-line ESCC Patients
The safety of TEVIMBRA in second-line ESCC treatment was evaluated in RATIONALE-302, a randomized, active-controlled, open-label, multicenter study in 255 patients with unresectable advanced, recurrent or metastatic ESCC. The trial excluded patients who had brain or leptomeningeal metastases that were symptomatic or required treatment, active autoimmune disease, a medical condition requiring systemic corticosteroids or immunosuppressants, or apparent tumor invasion of organs adjacent to the esophageal site.
Serious adverse reactions occurred in 41% of patients; the most frequent serious adverse reactions (≥2%) were pneumonia, dysphagia, hemorrhage, pneumonitis (including pneumonitis and immune-mediated pneumonitis), and esophageal obstruction. Fatal adverse reactions occurred in 7% of patients who received TEVIMBRA, including the following which occurred in more than one patient: pneumonia/pneumonitis (5 patients), hemorrhage (3 patients), and death due to an unknown cause (3 patients).
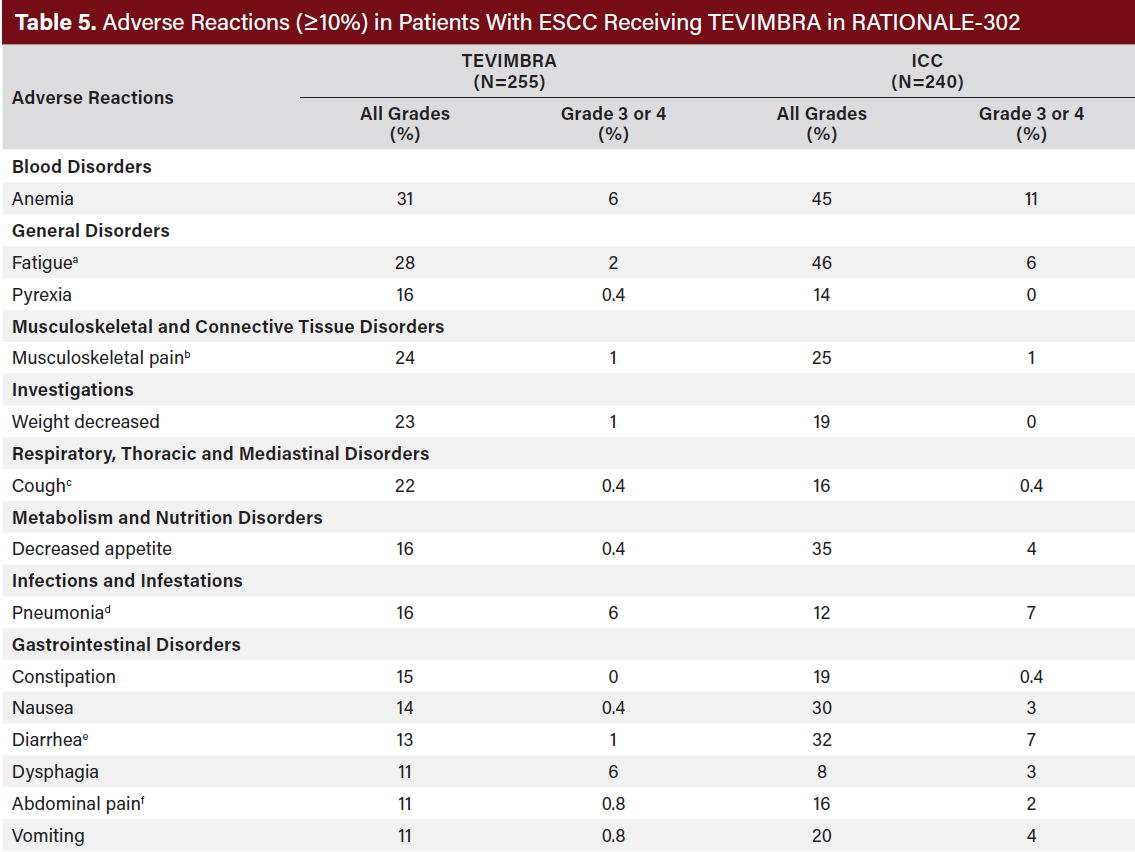
Permanent discontinuation of TEVIMBRA due to an adverse reaction occurred in 19% of patients. Adverse reactions which resulted in permanent discontinuation in ≥1% of patients were hemorrhage, pneumonitis (including pneumonitis and immune-mediated pneumonitis), and pneumonia.
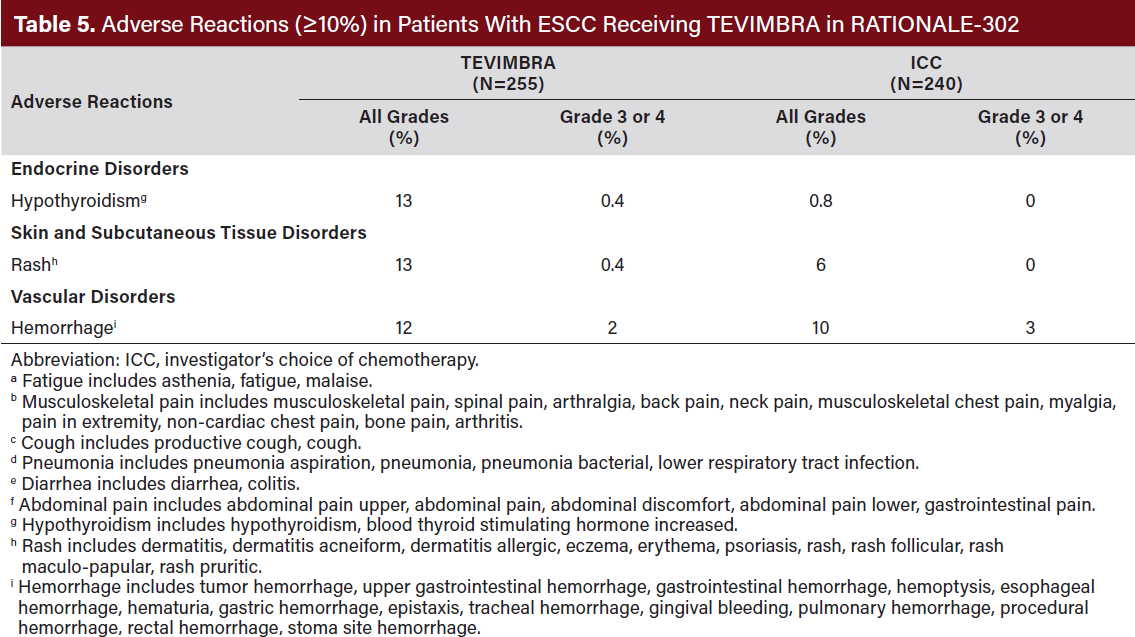
Dosage interruptions of TEVIMBRA due to an adverse reaction occurred in 23% of patients. Adverse reactions which required dosage interruptions in ≥2% of patients were pneumonia, pneumonitis, and fatigue. The most common (≥20%) adverse reactions were anemia, fatigue, musculoskeletal pain, decreased weight, and cough (Table 5, 6).
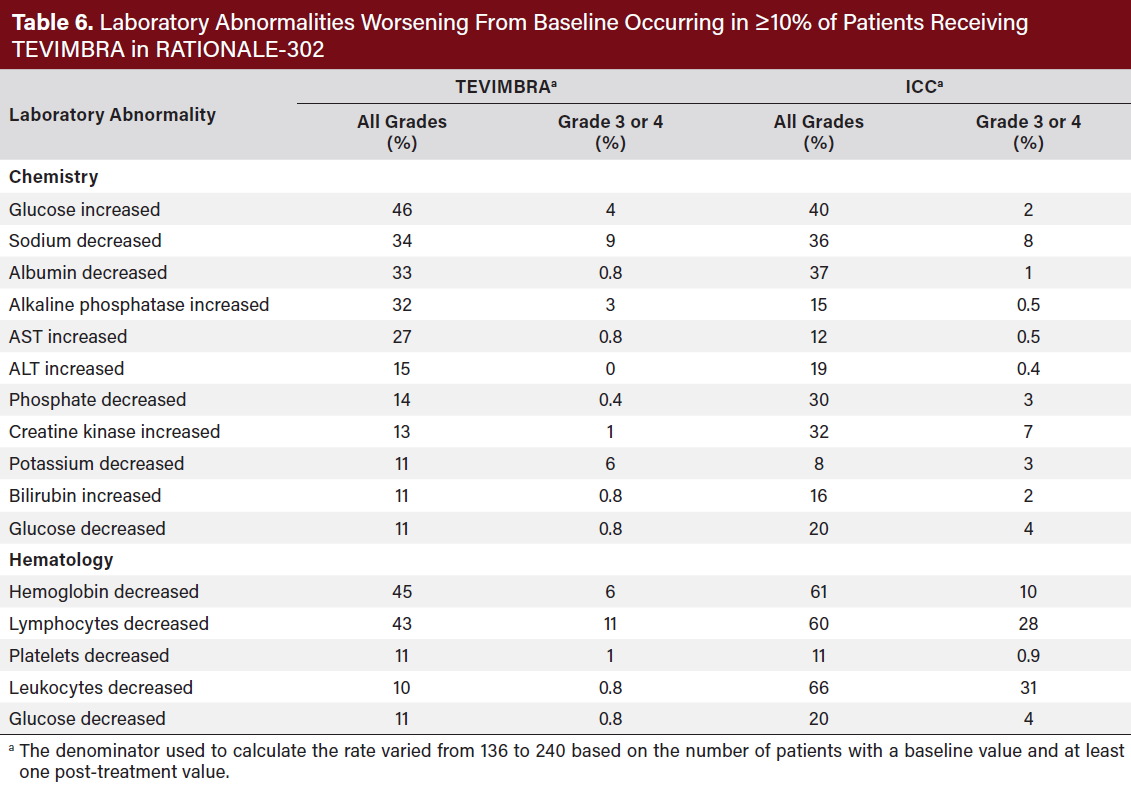
DOSAGE AND ADMINISTRATION
TEVIMBRA is available as a 100 mg/10 mL solution in a single-dose vial. TEVIMBRA should be stored in a refrigerator at 2°C to 8°C (36°F to 46°F) in the original carton to protect from light. The recommended dosage of TEVIMBRA as a single agent or in combination with platinum and fluoropyrimidine-containing chemotherapy is 150 mg every 2 weeks or 200 mg every 3 weeks or 300 mg every 4 weeks administered as an intravenous (IV) infusion until disease progression or unacceptable toxicity.
Prepare the solution for infusion as follows:
-
Withdraw 20 mL of TEVIMBRA from two vials of TEVIMBRA (for a total of 200 mg in 20 mL).
-
Transfer solution into an intravenous infusion bag containing 0.9% Sodium Chloride Injection, USP to prepare an infusion solution with a final concentration of 2 mg/mL to 5 mg/mL.
-
Mix diluted solution by gentle inversion to avoid foaming or excessive shearing of the solution. Do not shake.
-
TEVIMBRA is for single use only. Discard any unused portion left in the vial.
As TEVIMBRA does not contain any preservatives, if not used immediately, store the TEVIMBRA diluted solution either:
-
At room temperature at 20°C to 25°C (68°F to 77°F) for no more than 4 hours, including preparation and infusion duration. Discard after 4 hours.
-
Under refrigeration at 2°C to 8°C (36°F to 46°F) for up to 20 hours, including preparation and infusion duration. Allow the diluted solution to come to room temperature prior to administration. Discard after 20 hours.
Do not freeze the diluted solution.
Administer diluted solution by intravenous infusion through an intravenous line with a sterile, nonpyrogenic, low protein binding 0.2 micron or 0.22 micron in-line or add-on filter. For 150 mg and 200 mg doses, administer the initial infusion over 60 minutes. If tolerated, all subsequent infusions may be administered over 30 minutes. For 300 mg doses, administer the initial infusion over 90 minutes. If tolerated, administer the second infusion over 60 minutes. If the second infusion is tolerated, administer subsequent infusions over 30 minutes. Do NOT coadminister other drugs through the same infusion line. Do NOT administer TEVIMBRA asan intravenous push or single bolus injection. Flush the intravenous line at the end of infusion.
No dose reduction of TEVIMBRA is recommended. In general, withhold TEVIMBRA for severe (Grade 3) immune-mediated adverse reactions. Permanently discontinue TEVIMBRA for life-threatening (Grade 4) immune-mediated adverse reactions, recurrent severe (Grade 3) immune-mediated reactions that require systemic immunosuppressive treatment, or an inability to reduce corticosteroid dose to 10 mg or less of prednisone equivalent per day within 12 weeks of initiating steroids.
SUMMARY
Tislelizumab-jsgr is a novel anti–PD-1 antibody that demonstrates improved PD-1 binding characteristics compared with previously approved anti–PD-1 antibodies. In adult patients with unresectable or metastatic ESCC, tislelizumab-jsgr – in combination with platinum-containing chemotherapy and as a single agent after prior systemic chemotherapy that did not include a PD-(L)1 inhibitor – demonstrated a significant improvement compared with placebo in overall survival, progression-free survival, objective response rate, and duration of response. This suggests that tislelizumab-jsgr may be an effective addition to the treatment arsenal for patients with ESCC.













