Remote Therapeutic Monitoring to Support Adoption of Bispecific Antibody Therapy in Community Practices
Abstract
Targeted therapies have transformed the cancer treatment landscape, marking a paradigm shift in cancer treatment. Bispecific antibodies (BsAbs), which engage two antigens simultaneously, represent the next advancement in targeted immuno-oncologic treatment. However, as treatment modalities become more sophisticated so do their associated toxicities, necessitating more time-intensive and specialized approaches to toxicity management. This results in increased efforts by clinics to monitor patients, particularly in the absence of trainees or specialized staff, further adding to the cost of care associated with such advanced treatments. Remote therapeutic monitoring (RTM) using electronic patient-reported outcomes (ePROs) is a key component to cost-effective toxicity management for promising drugs such as BsAbs. It may enable greater access to BsAbs by reducing the burden of otherwise manual patient monitoring and symptom management.
This manuscript reviews the use of BsAbs, barriers to their adoption in community clinics, and how RTM can streamline and support patient management to improve patient outcomes and reduce the cost of adopting these novel treatments.
Background
Targeted immunotherapies are revolutionizing the treatment of hematologic malignancies, using the immune system to kill malignant cells while sparing normal cells. The next step in addressing the complexity of malignancy has been the introduction of bispecific antibodies (BsAbs), which have two targets.
Currently, most BsAbs are T-cell redirecting BsAbs that induce endogenous T-cells to target malignant cells expressing a uniquely expressed antigen of interest. Future BsAbs’ mechanisms of action (MOA) could vary, however, and induce any of a great number of physiological effects. Though the present article focuses on T-cell engagers, many of the concepts presented here apply more broadly to BsAbs or other drugs with significant toxicity.
Because BsAbs are associated with idiosyncratic toxicities that can quickly escalate to life-threatening conditions, close patient monitoring is necessary for their use. However, close monitoring comes at a high and sometimes prohibitive cost and is therefore ripe for automation and support using remote therapeutic monitoring (RTM).
History and MOA
The first monoclonal antibody (mAb) to be approved was rituximab, which targets CD20 commonly expressed in B-cell malignancies. It is associated with an excellent safety profile while significantly improving outcomes in both aggressive and indolent B-cell malignancies.1,2 A second wave included next-generation mAbs, more targets (ie, anti-CD38 and anti-CD52), and antibody-drug conjugates.
The next era of immunotherapies in hematologic malignancies has evolved to include BsAbs and chimeric antigen receptor T-cell (CAR-T) therapies, which have garnered a variety of US Food and Drug Administration (FDA)-approved indications.
Context and Use
Unlike the potentially curative one-time CAR-T treatment given at major transplant centers, BsAbs require repeated dosing that often occurs in outpatient clinics. The flexibility to administer BsAbs in outpatient clinics stems from BsAbs’ adjustable step-up dosing to more slowly draw outside effects brought on by activation of endogenous T-cells. In contrast, CAR-T entails a single infusion of genetically engineered cells that leads to potentially greater severity of toxicities.
The first approved BsAb, blinatumomab, a CD19 × CD3 T-cell engager for acute lymphocytic leukemia, is administered via continuous infusion, and initially requires inpatient monitoring—three days for cycle one and two days for the second 28-day treatment cycle.3
Since blinatumomab was approved, BsAbs have been approved for B-cell lymphoma4 and multiple myeloma,5 as well as uveal melanoma,6 small cell lung cancer,7 and non–small cell lung cancer8 (Table 1).
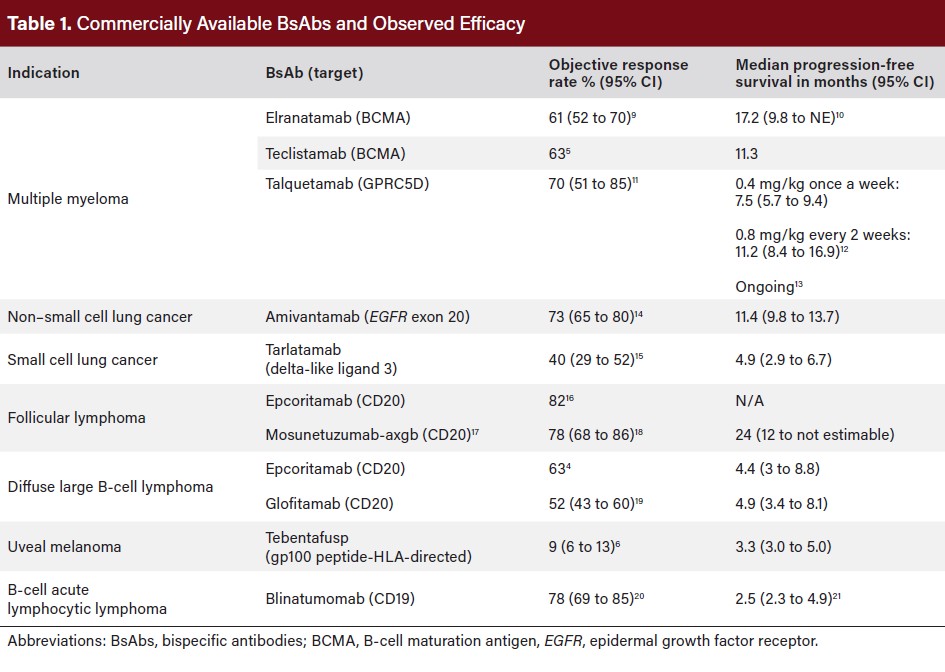
Administration
BsAb therapies require step-up dosing via intravenous route or subcutaneous injection to reduce severe short-term toxicities like cytokine release syndrome (CRS) and neurotoxicity, which are more pronounced in CAR-T therapy due to its single-dose administration. Still, all approved BsAbs carry a significant risk of acute toxicities, necessitating labeled protocols for step-up dosing, gradually increasing doses until full therapeutic dosing is reached.
Potential Toxicities
T-cell engaging BsAbs are associated with early toxicities related to activation of endogenous T-cells as well as ongoing toxicities related to potential on-target, off-tumor effects (Table 2). The most important acute toxicity is cytokine release syndrome (CRS), defined by a constellation of fever along with the possibility of varying degrees of hypotension and hypoxia.22
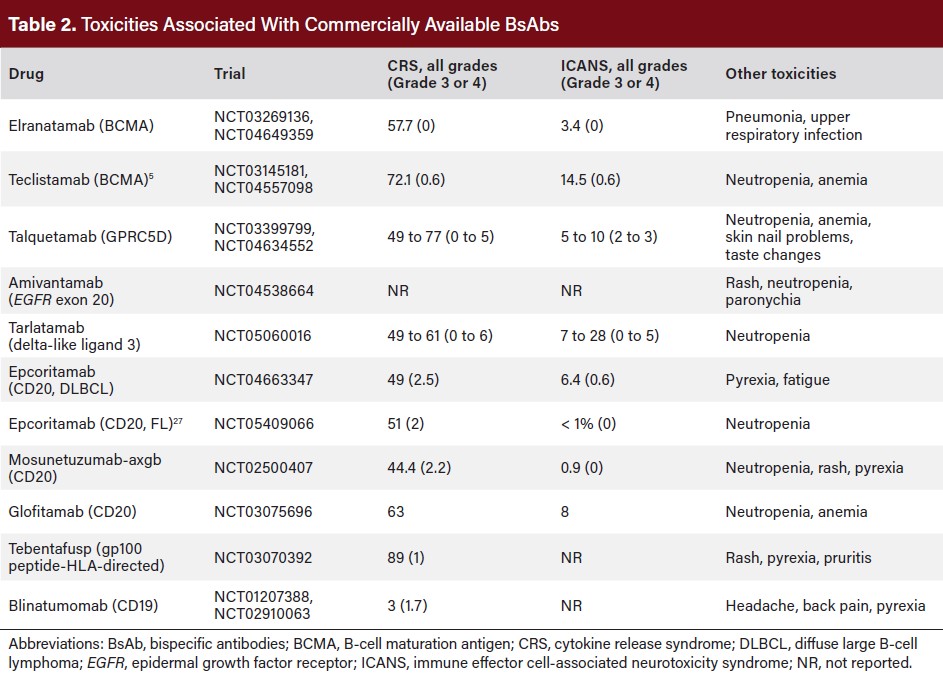
CRS is a well-known phenomenon since the advent of CAR T-cell therapy, with defined grading systems and management algorithms. Low-grade CRS may be manageable in both inpatient and outpatient settings with acetaminophen and dexamethasone, but high-grade CRS necessitates inpatient admission along with early use of anti-inflammatory agents such as tocilizumab and/or corticosteroids.23
Unlike CAR T-cell therapy, immune effector cell-associated neurotoxicity syndrome (ICANS) is relatively rare with BsAbs. However, it remains an important toxicity to monitor due to its potential impact on patient safety. ICANS occurs due to excessive immune response or cytokine release and can manifest as mild symptoms such as confusion or aphasia, up to severe symptoms such as seizure, cerebral edema, or even coma.
BsAbs for hematologic malignancy indications, especially BCMA and CD20-directed ones, are associated with a high risk of high-grade and/or opportunistic infections throughout their administration.24 Supportive care with intravenous immunoglobulins25 may help to reduce the risk of serious infections. Infections typically occur once the patient has already made it through the early toxicities and may not be monitored as closely; this suggests a need for remote monitoring platforms that are flexible to adapt to changing toxicities over time.
As more targets for BsAbs come forward, new on-target, off-tumor effects will emerge. For instance, the GPRC5D × CD3 BsAb talquetamab is associated with a high rate of significant skin changes such as desquamation, dystrophic nails and dysgeusia, and weight loss.11-13 The epidermal growth factor receptor-mesenchymal-epithelial transition (EGFR-MET) bispecific monoclonal antibody amivantamab is associated with severe rash in 3.9% of patients, although it does not lead to T-cell related toxicities.26
Early identification of symptoms may help to better manage the frequency and intensity of therapy along with guiding supportive care measures—a goal that ePROs support.
Standard of Care and FDA Label-Recommended Management
Due to the risk of CRS during step-up dosing, the FDA has at times included language in package inserts that “patients should be hospitalized” after each step-up dose administration. Some practices have interpreted “should” as a requirement for inpatient administration of BsAbs, while others have taken it as a recommendation.
A recent study of practice patterns regarding the BsAb teclistamab found that there were institution-level and patient-level factors that influenced outpatient vs inpatient step-up dosing.28 At the institutional level, inpatient bed availability, lack of reimbursement for outpatient tocilizumab, and interpretation of the package insert regarding the necessity for inpatient monitoring all impacted the decision to proceed with inpatient step-up dosing. In some cases, the lack of reimbursement for the inpatient step-up doses of drugs influenced practices’ ability to start treatment altogether. Notably, availability of dedicated staff for remote monitoring of symptoms also influenced the decision regarding treatment setting for step-up dosing. At the patient level, comorbid conditions, high disease burden, distance from a practice, caregiver availability, and insurance influenced their decision-making. Despite these challenges, all respondents indicated a desire to move toward outpatient step-up dosing, with the necessary tools available to do so safely.28
A major consideration in shifting step-up dosing to a fully outpatient model, particularly to community practices, is ensuring that suspected CRS cases can be effectively triaged and managed without compromising patient safety. Some practices have 24/7 urgent care capabilities, where supportive medications can be administered.29 Outpatient tocilizumab may be available, though it is more likely to be reimbursed if a practice includes it in the initial authorization for step-up dosing. Other practices have dedicated triage pathways through the emergency department to facilitate early corticosteroids or tocilizumab. Others employ remote monitoring among outpatients to help trigger inpatient admissions.30
With support from the Lymphoma Research Foundation, a panel of experts in BsAb therapy for lymphoma developed consensus guidelines on managing BsAb toxicity.25 The recommendations focus on CRS, covering pretreatment testing, patient education, monitoring, treatment settings, pre-medications, management, dose modifications, and retreatment. Other toxicities, including neurological events, tumor flare, cytopenia, infections, and tumor lysis syndrome, are also addressed.
Early identification of symptoms may help to better manage the frequency and intensity of therapy along with guiding supportive care measures—a goal that ePROs support.
Barriers to Bispecific Adoption in Community Oncology
BsAbs have generated a tremendous amount of excitement, but there have been barriers to widespread adoption. The novelty and severity of BsAb toxicities have posed substantial challenges to implementation for practices. They are as follows.
1. Physician and Nurse Education
Community oncologists routinely adopt novel therapies with unique toxicity profiles. The rapid integration of programmed cell death 1 (PD-1)/programmed cell death ligand 1 (PD-L1) inhibitors in solid tumor treatment31 demonstrated the ability of community oncologists to embrace effective new therapies. This quick adoption of immune checkpoint inhibitors occurred due to the early identification and recognition of new immune mediated toxicities and early education on their appropriate management.
Ultimately, this important education has been codified by the National Comprehensive Cancer Network (NCCN) and American Society of Clinical Oncology (ASCO) consensus guidelines.32,33 Similar guidelines will be needed for BsAbs. Initially, since the FDA labelled indications for these treatments are limited, it may be wise to restrict the community practice sites/team to a smaller group of physicians and nurses with special expertise. However, as the number of indications for BsAbs grow, so will the need to increase the number of sites who can manage patients on BsAb treatment.
2. Optimized Monitoring and Response Protocols
BsAb toxicities often follow predictable time frames, and monitoring should align with these timelines. CRS and ICANS typically occur after the initial or step-up doses, while infection risk increases with prolonged immunosuppression.
Due to the frequency and severity of CRS, most practices hospitalize patients after administration for close observation and intervention. However, given the low frequency of grade 3-4 CRS, most may be unnecessary. With a dedicated, well-trained team conducting structured remote assessments, some hospitalizations could be avoided.
Current remote monitoring involves thermometers, pulse oximeters, and blood pressure cuffs, supplemented by nurse-led check-ins every 4 hours, with modifications for overnight rest. During office hours, an experienced team, led by the managing physician, is available for immediate intervention. However, this labor-intensive model is costly and unsustainable as patient volumes increase.
A technology-driven approach to remote therapeutic and physiologic monitoring could improve efficiency, standardize post-treatment care, and reduce unnecessary hospitalizations as well as the cost of this expensive therapy.
3. Consistent Inpatient Admission Availability
Safe BsAb treatment requires accessible outpatient facilities and a reliable hospital partner. While outpatient infusion is rarely an issue, inpatient admission or post-infusion observation requires bed availability. If inpatient treatment or post-infusion observation is needed, hospital bed availability is essential.
For patients managed remotely, guaranteed hospital access is critical for overnight toxicity events or escalating conditions. Securing a hospital partner can be challenging, as reimbursement concerns may deter hospitals from administering bispecifics. Emergency room physicians and hospitalists often have limited experience with CRS. Delayed recognition and treatment of a febrile bispecific patient in the ER could lead to serious complications. Hospital staff must be educated on toxicity management, provided with clear evaluation and treatment protocols, and equipped with essential supportive care agents, along with ICU-level monitoring capabilities.
4. Financial Considerations
Treating patients with BsAbs requires significant physician and staff time, much of which is uncompensated. The added work of ensuring patients are well-monitored can be addressed by residents or fellows at teaching hospitals, but at community oncology clinics trainees are generally not available to raise concerns. For BsAbs to be feasible in community oncology clinics, either the cost of manual monitoring must be reimbursed, or monitoring must be made less expensive. Both options can be addressed by RTM because it is reimbursable and it automates a variety of tasks.
Additionally, practices often provide essential monitoring equipment (thermometers, pulse oximeters, blood pressure cuffs) at their own expense, as these items are not covered by insurance. Most of these expenses are upfront, incurred during the first couple of weeks of treatment and well before any reimbursement for the care delivered.
Community oncologists are typically reimbursed for injectable anti-cancer drugs based on the average sales price (ASP) plus a percentage. This added margin helps offset the overhead of chemotherapy administration, but BsAb reimbursement does not always follow this model. Medicare provides the most straightforward reimbursement for bispecifics, requiring no prior authorization and paying ASP + 6%.34 In contrast, commercial insurers often require prior authorization and may base reimbursement on invoice price or other negotiated rates, creating financial uncertainty.
Given the inadequate reimbursement for BsAbs and other high-cost therapies, many community practices may find administering these agents financially unviable.
Technology-Enabled Solutions
Many of the barriers associated with BsAb adoption could be addressed if clinical teams could follow patients more closely after administration. RTM represents one such solution.
Studies have demonstrated that ePRO-based RTM is associated with an increased rate of symptom detection by the care team, reduced occurrence of hospitalizations,35 improved health-related quality of life, increased time on treatment,36 and, in some studies, even increased overall survival,37 among other benefits.
Regarding BsAbs specifically, data from Canopy Care shows that RTM identifies BsAb-related symptoms up to three weeks earlier than traditional phone-based follow-up. Nearly all symptoms were reported more frequently by ePRO compared to phone, including essential symptoms to detect during BsAb treatment, such as fever, headache, and others.38
RTM provides a meaningful opportunity to address barriers to BsAb adoption by enabling symptom surveillance, standardizing toxicity management, streamlining clinician workload, and enhancing patient engagement. A detailed breakdown of these barriers and how RTM can address them are provided below.
1. Physician and Staff Education
As discussed above, clinicians and staff must be well-versed in recognizing and managing BsAbs toxicities such as CRS and ICANS. Given the ubiquity of these toxicities across the class and the intensive protocols associated with dealing with them, effective use of BsAbs demands structured toxicity management strategies.
Using RTM, therapy-specific algorithms are implemented that (1) perform the initial triage, directing clinicians to the most urgent patient reports, and (2) arm them with the appropriate decision tree to best triage those symptoms. An array of visual cues (highlighting or coloring in red, for example) within the software are then used to direct disposition. Such approaches are already widely adopted in other areas of clinical practice—such as lab reports that highlight findings that are out of range—and are ripe for implementation as part of therapy-specific RTM.
With information about the patient’s state in hand, clinicians are faced with decisions about symptom management. Though decision support systems currently focus on treatment selection, guidelines around symptom management can be provided within decision support systems specific to oncology and RTM. Rather than sifting through the literature on the spot, relevant information about symptom management can be provided concurrently with patient review.
2. Optimized Monitoring and Response Protocols
In the absence of a technology-enabled solution, the monitoring recommendations for BsAbs are time-intensive and potentially burdensome. For example, consensus recommendations for CD3 × CD20 BsAbs suggest phone calls every few hours.39 RTM could automate this process, allowing patients to receive the requisite check-ins, while simultaneously enabling nurses to focus on patients who have known issues rather than spending valuable time collecting data from patients who are not experiencing actionable symptoms.
Because BsAb toxicities such as CRS and ICANS often follow predictable timelines, RTM assessments can be scheduled to align with those timelines and existing guidance. Patients can submit ePROs at predefined intervals, allowing for coordinated triage. Nurse teams reviewing these reports can classify responses into three categories: (1) no follow-up needed, (2) additional patient contact required for clarification, or (3) escalation to a physician for emergent concerns (Figure).
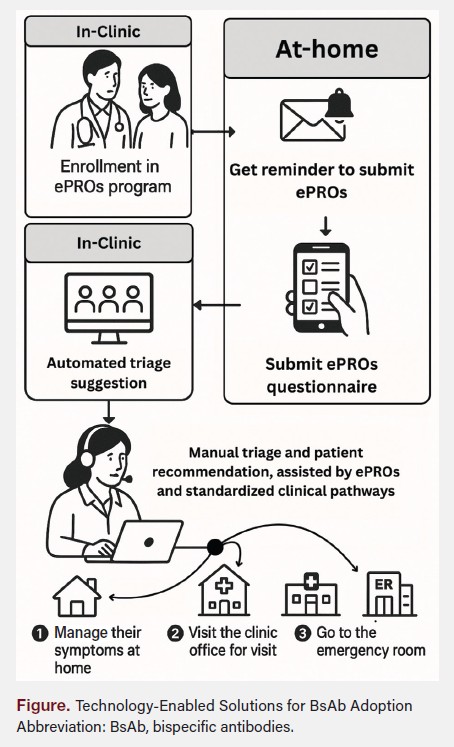
A structured remote monitoring approach ensures that critical toxicities are identified promptly without relying solely on patient-initiated reporting or manual follow-up processes. Automated triage algorithms can facilitate intervention earlier in the course of toxicity development, while decision support tools standardize optimal management, reducing the need for hospitalization.
3. Consistent Inpatient Admission Availability
Traditional monitoring relies on hospital admission for observation during step-up dosing, yet bed availability and hospital reimbursement concerns pose significant hurdles. In the face of BsAb administration increasingly shifting to an outpatient model, safe BsAb administration requires reliable access to inpatient care.
Previous work suggests that many acute cancer-related hospital encounters might be avoidable with earlier intervention40-42 and RTM. Applied at scale, an RTM system that facilitates such earlier interventions could reduce the use of hospital resources by patients receiving BsAbs as it has been shown to reduce hospitalizations,35 likely as a result of earlier toxicity detection and management, reducing the burden of BsAbs adoption to clinic-affiliated hospitals as well as overall costs of care.
The broad safety of a shift to outpatient BsAb treatment is further supported by recent efforts to create a reliable toxicity prophylaxis for BsAbs. For example, in the case of teclistamab, patients may receive prophylactic tocilizumab to minimize the risk of high-grade CRS, while steroids may be co-administered with BsAbs. It may be possible that steroids could be given to patients at home to take after reporting CRS symptoms by RTM, thus avoiding hospitalization.
Last, care coordination and transfer of care are their own challenges, too often leaving nurses to search for a BsAb-ready hospital with available beds. Within the framework of an RTM initiative, this could also be automated, again leading to quicker patient care and reduced clinician time spent on operational tasks that interrupt time spent with patients.
Using RTM and advances in treatment protocols will not eliminate the possibility of CRS, but it could lead to detection at earlier grades of symptom onset and reduce the burden of BsAb adoption to clinic-affiliated hospitals.
4. Financial Considerations
The uncompensated, “invisible” cost of time spent on symptom monitoring is a significant barrier limiting access to BsAbs and their adoption. RTM holds the potential to not only reduce these invisible costs but enable reimbursement for the associated care delivered.
Manual processes for toxicity monitoring, particularly phone calls and added follow-up, are labor-intensive and unsustainable at scale. RTM addresses these challenges by automating symptom tracking and triage, reducing the need for constant clinician oversight and helping to reduce the cost of care.
Moreover, RTM may recover some of the costs of monitoring: In the 2022 physician fee schedule, the Centers for Medicare & Medicaid Services (CMS) introduced five new RTM codes, significantly expanding reimbursement for remote care services.43 This recent development further opens the door to a sustainable financial model for community practices to offer BsAbs such that the operational costs of offering them is both reduced and offset by RTM.
The Future of RTM in BsAb Therapy
The integration of RTM into BsAb treatment workflows represents a significant advancement in patient care. But to realize these benefits, patient engagement remains a critical factor in RTM success. Seamless user interfaces, automated reminders, and proactive interventions for nonresponsive patients are essential to optimize adherence. Ensuring that patients understand the importance of regular symptom reporting and minimizing barriers to participation will be key to long-term adoption.
By addressing the primary challenges of BsAb therapy— education, monitoring, timely care access, and financial sustainability—RTM provides a scalable, effective solution for community oncology practices. As technology-enabled patient monitoring continues to evolve, RTM will play an increasingly central role in ensuring safe and efficient BsAb administration.
Conclusions and Next Steps
There is an urgent need to provide solutions to the challenges community oncologists face in the administration of BsAbs. These agents are an important addition to the therapeutic armamentarium for hematological malignancies and increasingly for advanced solid tumors.
The ability of a skilled medical team to monitor patients remotely to allow early detection and facilitate prompt, standardized treatment of toxicity will dramatically alleviate many of the current impediments.
ePRO technology is a transformative approach to patient monitoring, leading to significantly lower costs and increased efficiency. By facilitating early intervention and reducing the need for hospital stays, ePRO technology stands to improve patient outcomes and drive substantial cost savings.
This technology has several other potential benefits:
- Each BsAb has a unique toxicity profile, and ePRO platforms can easily be adapted to provide drug-specific monitoring with appropriate symptom management.
- Because monitoring protocols include management pathways, RTM technology should provide a turn-key approach to practice adoption of all but the most toxic therapies.
- By integrating clinical data, patient-reported outcomes, and health outcomes, ePROs-based RTM can enhance personalized treatment strategies. For example, real-world data collection could improve risk stratification for CRS, as current insights are based primarily on clinical trial data. Since relevant data already exist in patient records, real-world evidence can be leveraged to optimize care.
Remote monitoring of BsAbs treatment represents a paradigm for the use of patient-facing technology to facilitate safe administration of a novel, highly effective but potentially toxic therapy. Patients will benefit by having access to this life-saving treatment under the direction of their community oncologist.
Clinical Pathway Category: Infrastructure & Innovation
Integrating health care technology such as remote therapeutic monitoring (RTM) into clinical workflows supports the safe, scalable adoption of bispecific antibodies (BsAbs) in community oncology. Innovation through embedding digital tools into clinical pathways can help expand access to complex therapies beyond academic centers.
Author Information
Affiliations:
1Oncology Hematology Care, Cincinnati, Ohio; 2Department of Medicine, The University of Chicago; 3Texas Oncology; 4Canopy Care, New York, New York
Correspondence:
Mustafa Ascha
Canopy Care
228 Park Ave S, Suite 72460
New York, NY 10003-1502
mustafa@canopycare.us
Disclosures:
L.K. is the founder and CEO of Canopy Care. M.A., G.C., and M.K. are employed by Canopy Care. J.E. has received a speaker honorarium from Genmab, Inc. B.D. is a paid consultant for Canopy Care, COTA, Janssen Pharmaceuticals, and Sanofi, and is an independent reviewer for a clinical trial for Bristol Myers Squibb.
References
1. Coiffier B, Lepage E, Brière J, et al. CHOP chemotherapy plus rituximab compared with chop alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346(4):235-242. doi:10.1056/nejmoa011795
2. Hainsworth JD, Litchy S, Burris HA, et al. Rituximab as first-line and maintenance therapy for patients with indolent non-hodgkin’s lymphoma. J Clin Oncol. 2002;20(20):4261-4267. doi:10.1200/jco.2002.08.674
3. Kantarjian H, Stein A, Gökbuget N, et al. Blinatumomab versus chemotherapy for advanced acute lymphoblastic leukemia. N Engl J Med. 2017;376(9):836-847. doi:10.1056/nejmoa1609783
4. Thieblemont C, Karimi YH, Ghesquieres H, et al. Epcoritamab in relapsed/refractory large B-cell lymphoma: 2-year follow-up from the pivotal EPCORE NHL-1 trial. Leukemia. 2024;38(12):2653-2662. doi:10.1038/s41375-024-02410-8
5. Moreau P, Garfall AL, van de Donk NWCJ, et al. Teclistamab in relapsed or refractory multiple myeloma. N Engl J Med. 2022;387(6):495-505. doi:10.1056/nejmoa2203478
6. Nathan P, Hassel JC, Rutkowski P, et al. Overall survival benefit with tebentafusp in metastatic uveal melanoma. N Engl J Med. 2021;385(13):1196-1206. doi:10.1056/ nejmoa2103485
7. Ahn M, Byoung Chul Cho, Felip E, et al. Tarlatamab for patients with previously treated small-cell lung cancer. N Engl J Med. 2023;389(22):2063-2075. doi:10.1056/ nejmoa2307980
8. Syed YY. Amivantamab: first approval. Drugs. 2021;81(11):1349-1353. doi:10.1007/ s40265-021-01561-7
9. Lesokhin AM, Tomasson MH, Bertrand Arnulf, et al. Elranatamab in relapsed or refractory multiple myeloma: phase 2 MagnetisMM-3 trial results. Nat Med. 2023;29(9):2259-2267. doi:10.1038/s41591-023-02528-9
10. Tomasson MH, Iida S, Niesvizky R, et al. Long‐term survival and safety of elranatamab in patients with relapsed or refractory multiple myeloma: update from the MagnetisMM‐3 study. Hemasphere. 2024;8(7):e136. doi:10.1002/hem3.136
11. Chari A, Minnema MC, Berdeja JG, et al. Talquetamab, a T-cell–redirecting GPRC5D bispecific antibody for multiple myeloma. N Engl J Med. 2022;387(24):2232-2244. doi:10.1056/nejmoa2204591
12. Chari A, Touzeau C, Schinke C, et al. Safety and activity of talquetamab in patients with relapsed or refractory multiple myeloma (MonumenTAL-1): a multicentre, open-label, phase 1–2 study. Lancet Haematol. 2025;12(4):e269-e281. doi:10.1016/ s2352-3026(24)00385-5
13. Labanca C, Martino EA, Vigna E, et al. Talquetamab in multiple myeloma: efficacy, safety, and future directions. Eur J Haematol. 2024;114(3):386-399. doi:10.1111/ejh.14353
14. Zhou C, Tang KJ, Cho BC, et al. Amivantamab plus chemotherapy in NSCLC with EGFR Exon 20 Insertions. N Engl J Med. 2023;389(22):2039-2051. doi:10.1056/nejmoa2306441
15. Ahn M, Cho BC, Felip E, et al. Tarlatamab for patients with previously treated small-cell lung cancer. N Engl J Med. 2023;389(22):2063-2075. doi:0.1056/ nejmoa2307980
16. Linton KM, Vitolo U, Jurczak W, et al. Epcoritamab monotherapy in patients with relapsed or refractory follicular lymphoma (EPCORE NHL-1): a phase 2 cohort of a single-arm, multicentre study. Lancet Haematol. 2024;11(8):e593-e605. doi:10.1016/S2352-3026(24)00166-2
17. Matasar M, Bartlett NL, Shadman M, et al. Mosunetuzumab safety profile in patients with relapsed/refractory B-cell non-hodgkin lymphoma: clinical management experience from a pivotal phase I/II trial. Clin Lymphoma Myeloma Leuk. 2024;24(4):240-253. doi:10.1016/j.clml.2023.12.005
18. Sehn LH, Bartlett NL, Matasar MJ, et al. Long-term 3-year follow-up of mosunetuzumab in relapsed or refractory follicular lymphoma after ≥2 prior therapies. Blood. 2024;145(7):708-719. doi:10.1182/blood.2024025454
19. Dickinson MJ, Carlo-Stella C, Morschhauser F, et al. Glofitamab for relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. 2022;387(24):2220-2231. doi:10.1056/nejmoa2206913
20. Gökbuget N, Dombret H, Bonifacio M, et al. Blinatumomab for minimal residual disease in adults with B-cell precursor acute lymphoblastic leukemia. Blood. 2018;131(14):1522-1531. doi:10.1182/blood-2017-08-798322
21. Coyle L, Morley NJ, Rambaldi A, et al. Open-label, phase 2 study of blinatumomab as second salvage therapy in adults with relapsed/refractory aggressive B-cell non-Hodgkin lymphoma. Leuk Lymphoma. 2020;61(9):2103-2112. doi:10.1080/1042 8194.2020.1759055
22. Lee DW, Santomasso BD, Locke FL, et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transplant. 2019;25(4):625-638. doi:10.1016/j.bbmt.2018.12.758
23. Davis JA, Snyder J, Rice M, et al. Dexamethasone for the management of CRS related to teclistamab in patients with relapsed/refractory multiple myeloma. Blood Cancer J. 2025;15(1):32. doi:10.1038/s41408-025-01222-y
24. Reynolds G, Cliff ERS, Mohyuddin GR, et al. Infections following bispecific antibodies in myeloma: a systematic review and meta-analysis. Blood Ad. 2023;7(19):5898- 5903. doi:10.1182/bloodadvances.2023010539
25. Guido Lancman, Parsa K, Kotlarz K, et al. IVIg use associated with ten-fold reduction of serious infections in multiple myeloma patients treated with anti-BCMA bispecific antibodies. Blood Cancer Discov. 2023;4(6):440-451. doi:10.1158/2643-3230.bcd-23-0049
26. Chon K, Larkins E, Chatterjee S, et al. FDA approval summary: amivantamab for the treatment of patients with non–small cell lung cancer with EGFR Exon 20 insertion mutations. Clin Cancer Res. 2023;29(17):3262-3266. doi:10.1158/1078-0432. ccr-22-3713
27. Falchi L, Sureda Balari A, Leppä S, et al. Fixed-duration epcoritamab + R2 drives deep and durable responses in patients with relapsed or refractory follicular lymphoma: 2-year follow-up from arm 2 of the Epcore NHL-2 trial. Blood. 2024;144:342. doi:10.1182/blood-2024-200262
28. Derman BA, Roach M, Lin D, et al. Panel interview of oncology practices with emergent experience of teclistamab in the real world: the TecPIONEER Study. Curr Med Res Opin. 2024;40(6):1053-1058. doi:10.1080/03007995.2024.2352856
29. Furqan F, Bhatlapenumarthi V, Dhakal B, et al. Outpatient administration of CAR T-cell therapies using a strategy of no remote monitoring and early CRS intervention. Blood Adv. 2024;8(16):4320-4329. doi:10.1182/bloodadvances.2024013239
30. Sandahl TB, Soefje SA, Fonseca R, et al. Real-world safety and health care resource utilization of teclistamab under an outpatient model for step-up dosing administration. JCO Oncol Pract. Published online December 20, 2024. doi:10.1200/ op-24-00489
31. O’Connor JM, Fessele KL, Steiner J, et al. Speed of adoption of immune checkpoint inhibitors of programmed cell death 1 protein and comparison of patient ages in clinical practice vs pivotal clinical trials. JAMA Oncol. 2018;4(8):e180798. doi:10.1001/jamaoncol.2018.0798
32. Vaddepally RK, Kharel P, Pandey R, Garje R, Chandra AB. Review of Indications of FDA-approved immune checkpoint inhibitors per NCCN guidelines with the level of evidence. Cancers. 2020;12(3):738. doi:10.3390/cancers12030738
33. Schneider BJ, Naidoo J, Santomasso BD, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: ASCO guideline update. J Clin Oncol. 2021;39(36):4073-4126. doi:10.1200/jco.21.01440
34. Medicare Part B Drug Average Sales Price. cms.gov. Accessed March 25, 2025. https://www.cms.gov/medicare/payment/fee-for-service-providers/part-b-drugs/average-drug-sales-price
35. Patt DA, Patel AM, Bhardwaj A, et al. Impact of remote symptom monitoring with electronic patient-reported outcomes on hospitalization, survival, and cost in community oncology practice: the Texas Two-Step Study. JCO Clin Cancer Inform. 2023;7:e2300182. doi:10.1200/cci.23.00182
36. Basch E, Deal AM, Kris MG, et al. Symptom monitoring with patient-reported outcomes during routine cancer treatment: a randomized controlled trial. J Clinical Oncol. 2016;34(6):557-565. doi: 10.1200/JCO.2015.63.0830
37. Basch E, Deal AM, Dueck AC, et al. Overall survival results of a trial assessing patient-reported outcomes for symptom monitoring during routine cancer treatment. JAMA. 2017;318(2):197-198. doi:10.1001/jama.2017.7156
38. Derman BA, Essell JH, Kolodziej MA, et al. Electronic patient-reported outcome (ePRO) symptom monitoring for relapsed/refractory multiple myeloma in community settings, focusing on bispecific antibody therapy. Blood. 2024;144(Supplement 1): 5046-5046. doi:10.1182/blood-2024-206505
39. Crombie JL, Graff T, Falchi L, et al. Consensus recommendations on the management of toxicity associated with CD3xCD20 bispecific antibody therapy. Blood. 2024;143(16):1565-1575. doi:10.1182/blood.2023022432
40. Cracchiolo JR, Arafat W, Atreja A, et al. Getting ready for real‐world use of electronic patient‐reported outcomes (ePROs) for patients with cancer: a National Comprehensive Cancer Network ePRO Workgroup paper. Cancer. 2023;129(16):2441- 2449. doi:10.1002/cncr.34844
41. Lipitz-Snyderman A, Pfister D, Classen D, et al. Preventable and mitigable adverse events in cancer care: measuring risk and harm across the continuum. Cancer. 2017;123(23):4728-4736. doi:10.1002/cncr.30916
42. Panattoni L, Fedorenko C, Greenwood-Hickman MA, et al. Characterizing potentially preventable cancer- and chronic disease–related emergency department use in the year after treatment initiation: a regional study. J Oncol Pract. 2018;14(3):e176-e185. doi:10.1200/jop.2017.028191
43. Department of Health and Human Services, Centers for Medicare & Medicaid Services. Medicare and Medicaid programs; omnibus COVID-19 health care staff vaccination. Federal Register. Published 2021. Accessed March 25, 2025. https://public-inspection.federalregister.gov/2021-23972.pdf














