Operationalizing Weight-Based Dosing of Pembrolizumab: Opportunities and Challenges
Abstract
The high cost of cancer care is partly driven by the high price of key oncologic drugs, such as pembrolizumab, which are often administered as flat doses rather than the weight-based doses for which they were originally approved by the US Food and Drug Administration. Pharmacoeconomic experts have advocated for a return to weight-based dosing as a drug waste-minimization strategy and to reduce the cost of cancer care. However, this recommended shift may present significant workflow challenges. At the American Oncology Network (AON), a large multisite community oncology practice participating in the Enhancing Oncology Model (EOM) with Thyme Care, we implemented various strategies to influence weight-based dosing of pembrolizumab. Approximately 8% of EOM episodes involve pembrolizumab administration, of which 70% involve patients whose weight would allow for reduced vial usage with weight-based dosing. Following several technological and process improvements, about 45% all pembrolizumab administrations at AON are now dosed by weight. Key takeaways from this effort include optimizing uses for technology, streamlining workflow, educating physicians, and using live-tracking modeling to measure performance and calibrate ongoing effort against results.
Introduction
Cancer remains a leading cause of mortality in the US and a primary contributor to the rising costs of American health care, largely due to the costs of oncologic drugs. According to the National Comprehensive Cancer Network (NCCN guidelines, some drugs can be dosed either in a flat dose or dynamically on the basis of body surface area or weight. For instance, pembrolizumab, a widely used immune checkpoint inhibitor, is typically given at a flat dose of either 200 mg every 3 weeks or 400 mg every 6 weeks (the latter gained popularity during the COVID-19 pandemic).1-3 However, the clinical trials that led to its approval by the US Food and Drug Administration used a weight-based dose of 2 mg/kg every 3 weeks.4 According to the latest Medicare average sales price (ASP) files, pembrolizumab is reimbursed by Medicare at approximately $5800 per 100 mg vial; this single drug accounts for more than one-third of all Medicare Part B drug spending. Pharmacoeconomic experts have therefore advocated for a return to a weight-based dosing protocol, which is widely used in other countries, arguing that such an approach would be therapeutically equivalent while generating significant cost savings.2,4-6
In the fee-for-service landscape, physicians are not incentivized to consider weight-based dosing. Current care workflows and care architectures deter such personalized dosing. Pembrolizumab was first made available in both 50 mg and 100 mg vials, but once the flat dose was approved, the 50 mg vial was withdrawn from the US market (it is still available in other countries).5,6 Most electronic health records (EHRs) include order regimens that default to the currently popular flat dosing. Sometimes, even motivated physicians find it difficult or impossible to make a change directly. Moreover, guideline-issuing organizations, including the NCCN, have not explicitly stated equivalence between flat and weight-based dosing approaches across all cancer types.
Within a value-based care context, the opportunity to reduce cost through weight-based dosing is much more salient and immediate. For example, the US Centers for Medicare & Medicaid Services (CMS) Enhancing Oncology Model (EOM), launched in July 2023, aligns incentives between Medicare and practices to pursue high-quality care at a lower cost. EOM requires practices to take on downside risk, encouraging cost-saving strategies in oncology. To enable success, pharmacy interventions are evaluated along important criteria, including patient outcomes and safety, cost of drug, volume of use, degree of the intervention’s operational complexity, and practice economics. Against many of these criteria, the pembrolizumab weight-based dosing is an ideal target. The drug is not only very expensive, but it is also widely used across numerous settings. The payer savings to practice margin loss is also significant: for every $1 lost in practice margin, CMS saves approximately $19 in care costs. Furthermore, switching from flat dosing to weight-based dosing could reduce pembrolizumab costs by up to 25%, which would directly yield greater reimbursement for EOM practices through cost savings.7,8
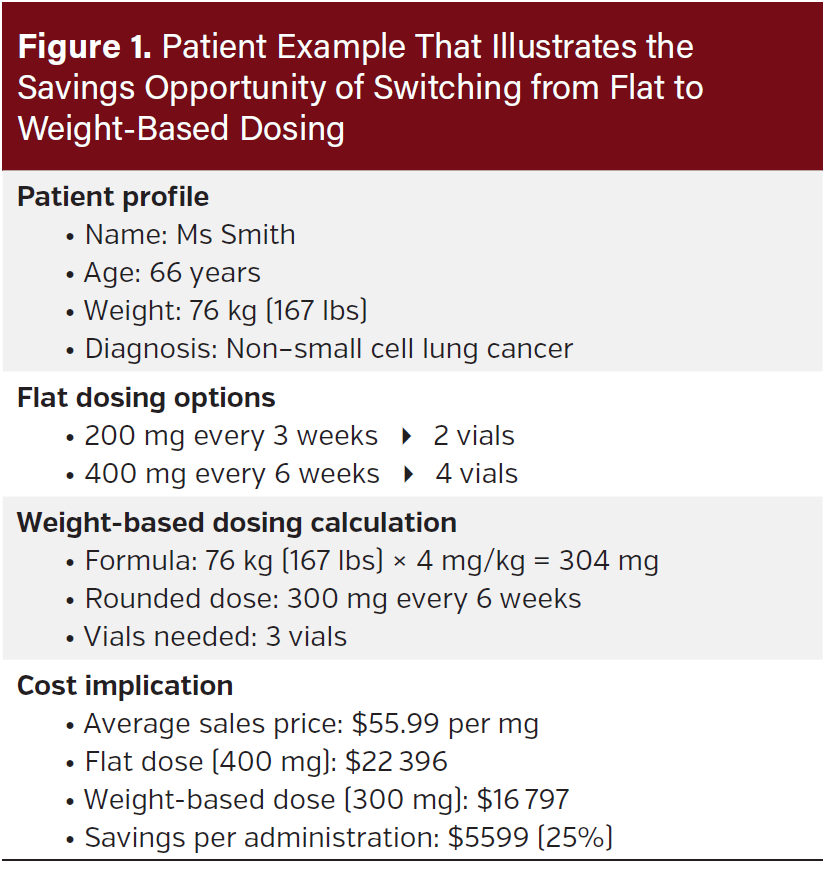
Here is a patient example. Ms Smith, a 66-year-old woman with non–small cell lung cancer, weighs 76 kg (167 lbs) and is receiving pembrolizumab. With flat dosing, she would receive either 200 mg (2 vials) every 3 weeks or 400 mg (4 vials) every 6 weeks. With weight-based dosing at 4 mg per kg of weight, her dose would be 304 mg every 6 weeks—rounded down to 300 mg, or 3 vials, every 6 weeks. This switch represents a 25% savings to the payer (Medicare) on this dose. At the current ASP pricing of $55.99 per mg, this equates to a savings of approximately $5599 per administration (Figure 1).
The operational lift of executing this intervention is difficult. In this article, we describe the various strategies used to influence prescribing of pembrolizumab weight-based dosing at the American Oncology Network (AON), a large multisite community oncology practice participating in the EOM with Thyme Care, a value-based care enablement company in cancer. Thyme Care provides AON with care management and navigation services, technical and data analytics support, advisory services, and actuarial and reinsurance services.
Methods
Our approach to transition from flat dosing to weight-based dosing was built on a comprehensive clinical pathway that integrates both process-oriented solutions and technological innovations. The initial data integration into AON’s data warehouse allowed clinical and data teams to review upcoming drug administrations and identify opportunities for intervention, yielding a 14% conversion rate from flat to weight-based dosing. There were no delays in obtaining insurance authorization for weight-based dosing vs flat dosing in a Medicare population, which does not require prior authorizations.
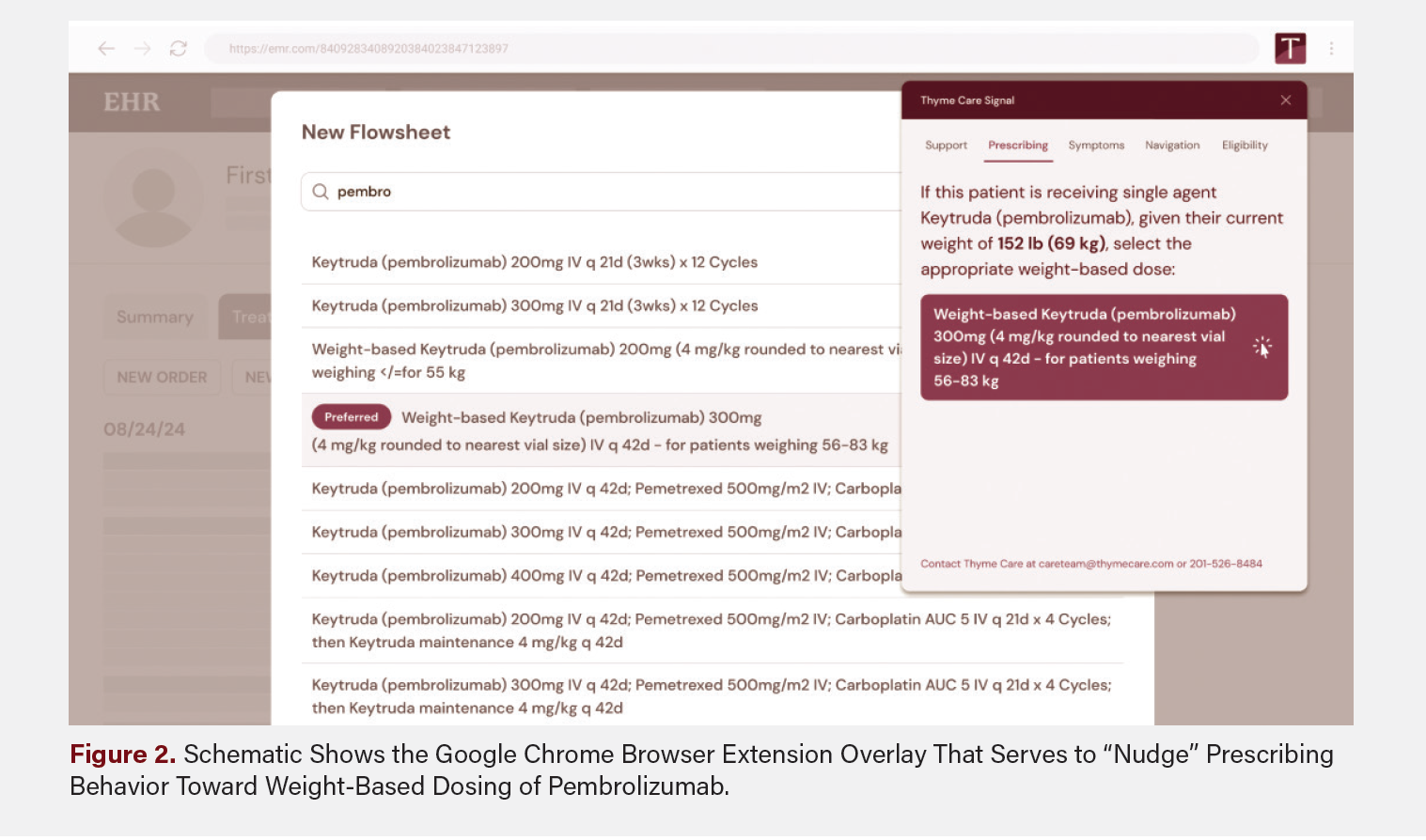
Recognizing the need for a more sustainable and scalable approach, we developed a multifaceted clinical pathway to embed this change directly into clinical workflows. We created two key technological innovations. First, we developed a Google Chrome browser extension that detects when physicians begin typing “pembro” or “keytruda” into the order section of the EHR and then triggers a real-time alert “nudge” to suggest modifying to weight-based dosing (Figure 2). Second, we collaborated with AON’s data and EHR support teams to create new regimens that inherently dose pembrolizumab by weight, with clear “EOM preferred” tags to guide selection.
We also implemented several complementary process strategies. We recommended that a pharmacy and therapeutics policy be created to automatically substitute flat doses of pembrolizumab to weight-based doses. This policy was strategically rolled out in phases, starting with new treatment-starts, then expanding to all single-agent pembrolizumab regimens, and finally to all patients regardless of newness or regimen type. We paired our technological interventions with comprehensive physician education and incorporated their feedback into the visual design of the nudges to prevent alert fatigue. Physician champions and regional medical directors were engaged to help promote the initiative and address any clinical concerns.
We identified that four pembrolizumab-containing regimens accounted for 75% of its overall use in the entire population, allowing us to target our efforts more efficiently. These regimens included two monotherapy regimens—pembrolizumab 200 mg every 3 weeks and 400 mg every 6 weeks—and two combinations for non–small cell lung cancer—pembrolizumab/ pemetrexed/carboplatin followed by either pemetrexed/pembrolizumab maintenance or pembrolizumab only maintenance. By focusing on these high-impact regimens, we ensured that our process and technology interventions would reach most cases requiring conversion to weight-based dosing.
The final component of our clinical pathway involves continuous auditing and monitoring. We dissect the success of the intervention by region, practice, and physician, and when we note heterogeneity, we institute learning sessions to improve and standardize workflows.
To operationalize this intervention, AON relied heavily upon their team of oncology clinical pharmacists who are distributed across their network and staffed based on region to support value-based care drug interventions. These pharmacists review clinical appropriateness for individual patients, update orders in the EHR, and coordinate patient communication and appointment scheduling with clinic staff. Because this intervention focuses on adjusting the dose and schedule of pembrolizumab, there were few or no changes to inventory management, and in this traditional Medicare population, resubmission of financial authorization was not necessary.
Results
Our analysis reveals that approximately 8% of EOM episodes involve pembrolizumab administration, with 70% of these episodes involving patients whose weight makes them eligible for reduced vial usage through weight-based dosing. Most pembrolizumab use occurs in patients with lung cancer (71%) and breast cancer (17%).
Since the launch of our initiative about 1 year ago, the combination of these different strategies has resulted in a conversion rate from flat to weight-based dosing of approximately 45% (up from 0%). Our internal threshold of success is to reach 75% of all eligible administrations. Each operational lever incrementally moves us closer to this goal. For example, our analytics show that the real-time clinical decision support tool, delivered through the Google Chrome browser extension, converts one-third of all pembrolizumab orders from flat to weight-based dosing by prompting oncologists at the point of prescribing to modify their order.
We calculate cost savings by comparing counterfactual standard dosing to actual intervention-based doses, using the CMS reimbursement formula (ASP + 6%, with CMS responsible for 80% and a 98% sequestration adjustment). To identify implementation patterns, we audit intervention uptake across physicians, practices, and regions, standardizing results as percentages of eligible administrations to help explain observed heterogeneity in adoption.
Following these technological and process improvements, 45% of all pembrolizumab administrations at AON are now weight-based. Each vial reduction saves Medicare approximately $5500 (accounting for quarterly ASP fluctuations). AON’s overall savings on this measure aligns with published data from other practices that have implemented this pharmacy intervention.9
Discussion
Our experience with pembrolizumab weight-based dosing across the initial performance periods ( July 2023 through June 2024) provides a blueprint for implementing cost-effective care initiatives in oncology practices nationwide. As health care continues to shift toward value-based models, several key insights emerge for organizations seeking to deploy similar interventions.
Future implementations should begin with establishing the right financial structure—specifically, a robust shared savings model that fairly compensates practices for both direct and indirect costs associated with intervention deployment. The return on investment must be meaningful enough to justify the significant operational lift required, acknowledging the delta between total drug cost savings (appreciated by payers) and the margin impacts for practices (dependent on procurement economics, rebates, and reimbursement structures). Equally important is clear and effective communication of the value proposition of the shared savings model: that practices are financially rewarded for reducing waste. This is essential to create the buy-in necessary to drive the cultural shift away from the status quo of costly flat dosing and suboptimal vial formulations.
Successful interventions require unified technological and clinical process integration, with the following elements:
- Embedded decision support systems: Tooling within the EHR should incorporate intelligent dosing recommendations directly within physician workflows, eliminating the need for separate programs or browsers.
- Proactive and cohesive policy frameworks: Pharmacy and therapeutics committees should consider, where possible, comprehensive policies that cover cost-effective dosing strategies across disease-agnostic and multiple use-case categories.
- Real-time analytics dashboards: Accessible, user-friendly analytics should enable clinical leaders to identify opportunities, track implementation, and address variation in real-time.
The sustainability of cost-effective care initiatives ultimately depends on cultural transformation. Future implementations should focus on cultivating the following:
- Physician champions: Influential clinicians can demonstrate how value-based interventions align with, rather than compete against, clinical excellence.
- Interdisciplinary collaboration: It’s vital to break down silos between pharmacy, clinical, and administrative teams to create solutions that work from multiple perspectives.
- Continuous learning systems: Keep the organization aligned and engaged by establishing regular forums for sharing successes, challenges, and innovations.
Key Challenges
Like many oncology practices, AON operates predominantly under a fee-for-service model. As such, multiple processes, from pharmacy procurement to measures of physician productivity, were all based on a fee-for-service paradigm. Among many of the clinical personnel, there was very little awareness of how value-based care programs were set up or what would be required of AON to shift toward this model. To address this, AON’s clinical and business leadership worked with Thyme Care to create multiple education opportunities for awareness, including presentations at pharmacy and therapeutics committees, frequent mentions in the chief medical officer’s newsletter, a permanent collection of video and one-page resources housed within an internal provider directory, and presentations at the AON’s large annual symposium.
Another common challenge with value-based care is the long delay between the end of a performance period and final reconciliation (approximately 13 months). This lag, needed to account for supplemental data submission and medical claims processing, means practices must wait to learn how they performed and whether they earned shared savings or owe recoupments to CMS. For the EOM, this means that a practice that started in July 2023, for example, would not receive its first shared savings payment until July 2025. To give AON real-time insight into its performance, Thyme Care’s data teams built a live-tracking model that created synthetic episodes, imputed unfinished costs, and leveraged a national cancer-specific trend ratio sourced from a data vendor. This work is essential to instilling confidence in practices that need to make significant financial investments, such as implementing weight-based pembrolizumab, without much immediate feedback on overall performance.
The last key challenge was resistance by providers who cited the lack of explicit inclusion of weight-based dosing of pembrolizumab in guidelines, such as NCCN, for all cancer types. Providers were concerned about possible liability associated with deviating from standard dosing paradigms. To address this, we sought to communicate the value proposition of our initiative through published studies demonstrating the equivalence of clinical efficacy of weight-based vs flat dosing of pembrolizumab, which was essential for instilling buy-in from hesitant providers.
Conclusion
Only a few practices nationwide participate in EOM, largely due to its financial risk and operational burden. Notably, AON accounts for nearly 10% of all EOM episodes. However, the principles of value persist in multiple other iterations, including Accountable Care Organizations, Integrated Provider Associations, and shared savings contracts with Medicare Advantage plans. While not all of these models are strictly capitated, they often incorporate population-based payments and risk-sharing mechanisms that align provider incentives with cost control and quality improvement.10-13
The next frontier of innovation will involve expansion from isolated interventions to comprehensive medication management strategies. Practices should build systems capable of simultaneously addressing multiple value opportunities across various therapeutic areas, creating economies of scale in their value-based pharmacy operations. While some speculate about mandating pharmacy policies, such as pembrolizumab weight-based dosing, this is just one example of a cost-saving intervention for payers that may reduce practice margin. Many similar opportunities are already implemented by payer-contracted utilization management vendors.
We believe there is a role for mandatory approaches, such as those seen with narrow formularies or step edits, in certain cases. However, models that financially incentivize oncologists to also actively consider the overall cost of care are more likely to unlock a broader range of opportunities without causing provider resistance and consternation about excessive insurer management.
Achieving this evolution will require payers and providers to develop more sophisticated partnerships—ones that recognize the significant operational investment needed and appropriately rewards practices for their role in delivering cost-effective care without compromising clinical outcomes or limiting appropriate therapeutic choices.
Clinical Pathway Category: Infrastructure & Innovation
The high cost of cancer care, particularly the high price of oncologic drugs, calls for a reevaluation of administering these drugs as flat doses vs weight-based doses. Clinical pathways can assist in the effort to optimize use of technology and streamline workflow to implement drug cost-saving strategies across practices.
Author Information
Affiliations: 1Thyme Care, Nashville, TN; 2Tennessee Oncology, Nashville, TN; 3University of Pennsylvania, Philadelphia, PA; 4American Oncology Network, Fort Myers, FL
Correspondence:
Samyukta Mullangi, MD, MBA
10 Lea Ave
Nashville, TN, 37210
Email: sam@thymecare.com
Acknowledgments: The authors would like to thank Brooke Peters, Maggie Murphy, Peter McMullan, and Robin Yoo for their contributions, support of the intervention, and critical review of this manuscript.
Disclosures: S.D. reports membership on pharmacy advisory boards at Daichi Sankyo, G1 Therapeutics, Inc, Johnson & Johnson, Caris Life Sciences, MiBA, American Oncology Network, and Pharmacosmos.
Availability of Data and Materials: The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
1. Goldstein DA, Ratain MJ, Saltz LB. Weight-based dosing of pembrolizumab every 6 weeks in the time of COVID-19. JAMA Oncol. 2020;6(11):1694-1695. doi:10.1001/ jamaoncol.2020.2493
2. Sehgal K, Costa DB, Rangachari D. Extended-interval dosing strategy of immune checkpoint inhibitors in lung cancer: will it outlast the COVID-19 pandemic? Front Oncol. 2020;10:1193. doi:10.3389/fonc.2020.01193
3. FDA approves new dosing regimen for pembrolizumab. US Food and Drug Administration. News release. April 28, 2020. Accessed June 16, 2025. https://www. fda.gov/drugs/resources-information-approved-drugs/fda-approves-new-dosing-regimen-pembrolizumab
4. Garon EB, Rizvi NA, Hui R, et al. KEYNOTE-001 Investigators. Pembrolizumab for the treatment of non–small-cell lung cancer. N Engl J Med. 2015;372(21):2018-2028. doi:10.1056/NEJMoa1501824
5. Sagonowsky E. Merck’s new keytruda flat dose could add $1B each year to U.S. healthcare spending. Fierce Pharma. News release. July 17, 2017. Accessed June 16, 2025. https://www.fiercepharma.com/pharma/keytruda-flat-dose-could-cost-extra-1b-each-year-for-u-s-healthcare-system-experts-say
6. Smeenk M, van der Noorth V, Hendrikx J, Kalkhorna H, Smit E, Theelen W. Pembrolizumab hybrid dosing is non-inferior to flat dosing in advanced non–small cell lung cancer: a real-world, retrospective bicenter cohort study. J Immunother Cancer. 2025;13(2):e010065. doi:10.1136/jitc-2024-010065
7. Slee A, Coutsouvelis J, Tong B, Poole S, Zalcberg J. A cost comparison of pembrolizumab: fixed and weight-based dosing. J Oncol Pharm Pract. Published online ahead of print May 23, 2024. doi:10.1177/10781552241255287
8. Goldstein D, Gordon N, Davidescu M, et al. A phamacoeconomic analysis of personalized dosing vs fixed dosing of pembrolizumab in firstline PD-L1-positive non– small cell lung cancer. J Natl Cancer Inst. 2017;109(11). doi:10.1093/jnci/djx063
9. Fernandez J, Indurlal P, Garey JS, Wilfong LS. Financial impact of fixed-dose versus weight-based dosing approaches for pembrolizumab and nivolumab in the Oncology Care Model for The US Oncology Network (The Network). J Clin Oncol. 2024;42(suppl 16). doi:10.1200/JCO.2024.42.16_suppl.e13573
10. Kyle M, Keating N. The promise and perils of oncology care in Medicare advantage. JAMA Network Open. 2024;7(9):e2434650. doi:10.1001/jamanetworkopen.2024.34650
11. Shah Z, Pandey S, Patel K, Dugar R. Payer management is coming for oncology drugs: what you should know. ZS. February 28, 2022. Accessed June 16, 2025. https://www.zs.com/insights/payer-management-is-coming-for-oncology-drugs
12. Mehta A, Macklis R. Overview of accountable care organizations for oncology specialists. J Oncol Pract. 2013;9(4):216-221. doi:10.1200/JOP.2012.000760
13. Sanghavi D, Patel K, Samuels K, et al. Transforming cancer care and the role of payment reform. Brookings Institute. August 26, 2014. Accessed June 16, 2025. https:// www.brookings.edu/articles/transforming-cancer-care-and-the-role-of-payment-reform/















