Avantec Vascular, a NIPRO Company, Announces FDA 510(k) Clearance of DVT-Focused Thrombectomy System
Avantec Press Release
Avantec Vascular, a NIPRO Company, has received FDA 510(k) clearance for a thrombectomy system that will enable a new type of treatment for patients with deep venous thrombosis (DVT).
Impact
It is estimated that more than 1 million people in the United States and 50 million people worldwide suffer from venous thromboembolism (VTE), which includes DVT. VTE is the third most common vascular disease after heart attack and stroke.
Application
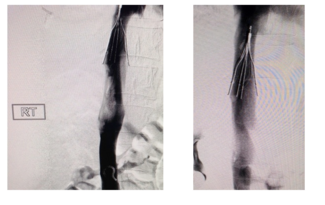
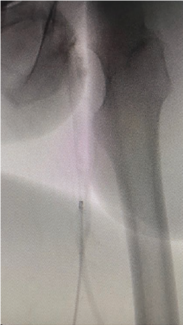
In the United States, this new thombectomy system is 510(k) cleared (K251207) with an indication for use in the “removal of fresh soft emboli and thrombi from vessels of the peripheral venous system > 7mm in diameter”.
Endovascular procedures with a minimally invasive percutaneous approach have become the most common method of treatment for patients with VTE to improve clinical symptoms over traditional surgical methods. Percutaneous techniques to mechanically disrupt, displace and remove thrombus within the venous system have been shown to be complementary to anti-clotting medication.
Method and Potential Benefits
This new thrombectomy system uses mechanical and aspirational methods of action to remove clot and emboli. With its utilization of a novel rotating tip mechanism combined with vacuum aspiration, the device is designed to be more versatile and efficient in removing a wide variety of blood clots, potentially shortening procedure time. Stabilization of the vein and increased blood flow are expected to improve patient outcomes.
"This new thrombectomy system may expand the range of venous thrombus subtypes that can be treated in a single session by combining aspiration and maceration functions in one device with a favorable profile," said Sirish Kishore, MD, Clinical Assistant Professor, Department of Radiology, Stanford University, and advisor to Aventec Vascular. "Preclinical data are encouraging, and I look forward to the results of the upcoming clinical studies."
Timing and Market Impact
Avantec is poised to begin bringing the clinical benefits of this device to U.S. patients through an initial limited market launch to key physician partners in 2026, with larger scale commercialization to follow. The treatment clearance of DVT represents an estimated $0.8B market opportunity in the United States.
About Avantec
Avantec, a NIPRO Group company, is committed to medical device innovation in the vascular and endovascular space, aligning with Nipro Medical Corporation to bring patients in the United States and across the globe simple, innovative solutions to complex issues affecting their vascular health.