The New Stedi Extra Support Guidewire, Designed for Use in TAVR
© 2026 HMP Global. All Rights Reserved.
Any views and opinions expressed are those of the author(s) and/or participants and do not necessarily reflect the views, policy, or position of Cath Lab Digest or HMP Global, their employees, and affiliates.
Tanvir K. Bajwa, MD, FACC, FSCAI
Cardiology Director, Structural Heart Program, Advocate Health St. Luke’s Medical Center, Milwaukee, Wisconsin
Disclosure: Dr. Bajwa reports a financial relationship with Medtronic as a speaker and proctor.
Dr. Tanvir K. Bajwa can be contacted at tanvir.bajwa@aah.org.
Click here for a PDF of this article, courtesy of Cath Lab Digest.
 Can you share some info about your experience with Evolut and your TAVR program?
Can you share some info about your experience with Evolut and your TAVR program?
I am the Cardiology Director of the Structural Heart Program at Advocate Health St. Luke’s Medical Center in Milwaukee, Wisconsin. Our program has been involved in all of the CoreValve/Evolut trials for evaluation of high, intermediate, and low-risk patient populations. Our program treats approximately 700 transcatheter aortic valve replacement (TAVR) patients a year and has implanted over 4,000 Evolut (Medtronic) TAVRs.
What aspects specific to Evolut valve delivery or deployment require extra attention from operators, or have proven to be challenging?
The Evolut valve delivery is reproducible when the cusp overlap and commissural alignment best practices are followed. Utilizing the steps of flush port at 3 o’clock, using the cusp overlap angle for deployment after ensuring the hat marker is at center-front for commissural alignment, and aiming for 3 mm depth makes challenging cases more predictable.
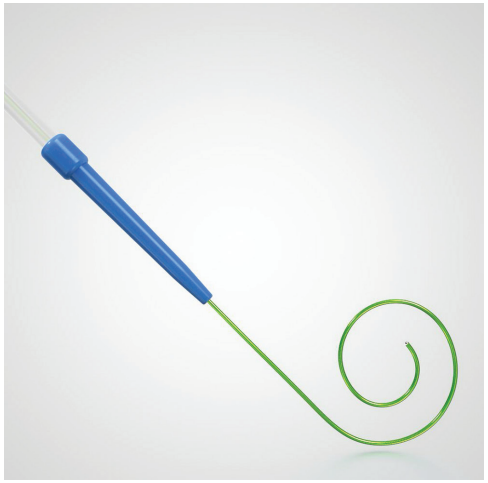
Can you describe the new Stedi Extra Support Guidewire design? How will it help during the TAVR procedure?
The Evolut FX delivery system has a single spine and coupled with the Stedi guidewire (Medtronic), provides a synergy that allows for more predictability with tracking throughout the aorta, and provides stability and predictability for deployment depth and coaxiality. Medtronic has a strong focus on procedure simplification and the Stedi wire contributes significantly to this goal.
What is cusp overlap technique and how does it benefit from the Stedi guidewire?
Cusp overlap technique isolates the non-coronary cusp of the aortic valve, which is the lowest cusp of the valve to achieve our target implant depth. The depth of deployment on the non-coronary cusp decreases the pacemaker rate, based on Medtronic Optimize PRO study data. The Stedi guidewire helps to achieve ideal placement of the delivery system and ultimately the valve between the non-coronary cusp and right coronary cusp commissure, which helps to achieve appropriate depth of valve implant and in turn, decreases pacemaker rates.
It’s early in use, but can you describe patient outcomes in procedures where the Stedi wire was used?
The unique design of the Stedi with the softness of the distal curve makes the ventricular component of the wire safe, despite its stiff main body. The main body support has helped us to achieve predictable deployments that are coaxial due to the way that the wire stays positioned during the Evolut deployment. Additionally, the design of the Stedi has allowed us to switch to utilizing only 1 wire per TAVR case, therefore saving money by cutting down on supply usage.
How was incorporating the Stedi into procedures for the TAVR team? What training was required?
We had a brief team in-service by the Medtronic team.
What patient anatomies or procedural situations might be appropriate for the Stedi wire?
I am currently utilizing it for all of my TAVR implants with all valve brands. I think that it is particularly impactful for 34 Evolut Valves and transcatheter aortic valve-in-surgical aortic valve (TAV in SAV) cases due to the stability that it provides. I also feel that could be impactful in mitral cases.