Artificial Intelligence for Chronic Total Occlusion Percutaneous Coronary Interventions
© 2025 HMP Global. All Rights Reserved.
Any views and opinions expressed are those of the author(s) and/or participants and do not necessarily reflect the views, policy, or position of the Journal of Invasive Cardiology or HMP Global, their employees, and affiliates.
Abstract
Artificial intelligence (AI) has become pivotal in advancing medical care, particularly in interventional cardiology. Recent AI developments have proven effective in guiding advanced procedures and complex decisions. The authors review the latest AI-based innovations in the diagnosis of chronic total occlusions (CTO) and in determining the probability of success of CTO percutaneous coronary intervention (PCI). Neural networks and deep learning strategies were the most commonly used algorithms, and the models were trained and deployed using a variety of data types, such as clinical parameters and imaging. AI holds great promise in facilitating CTO PCI.
Introduction
Advances in artificial intelligence (AI) are revolutionizing the landscape of medical care. In interventional cardiology, algorithmic developments can enable timely and accurate diagnosis, predict success and complications of complex procedures, and guide clinical decision-making using patient data and lesion characteristics. Despite recent progress, chronic total occlusion (CTO) percutaneous coronary intervention (PCI) is complex, costly, and could benefit from machine learning algorithms for prediction of outcomes and procedural guidance.
Overview of Machine and Deep Learning Algorithms
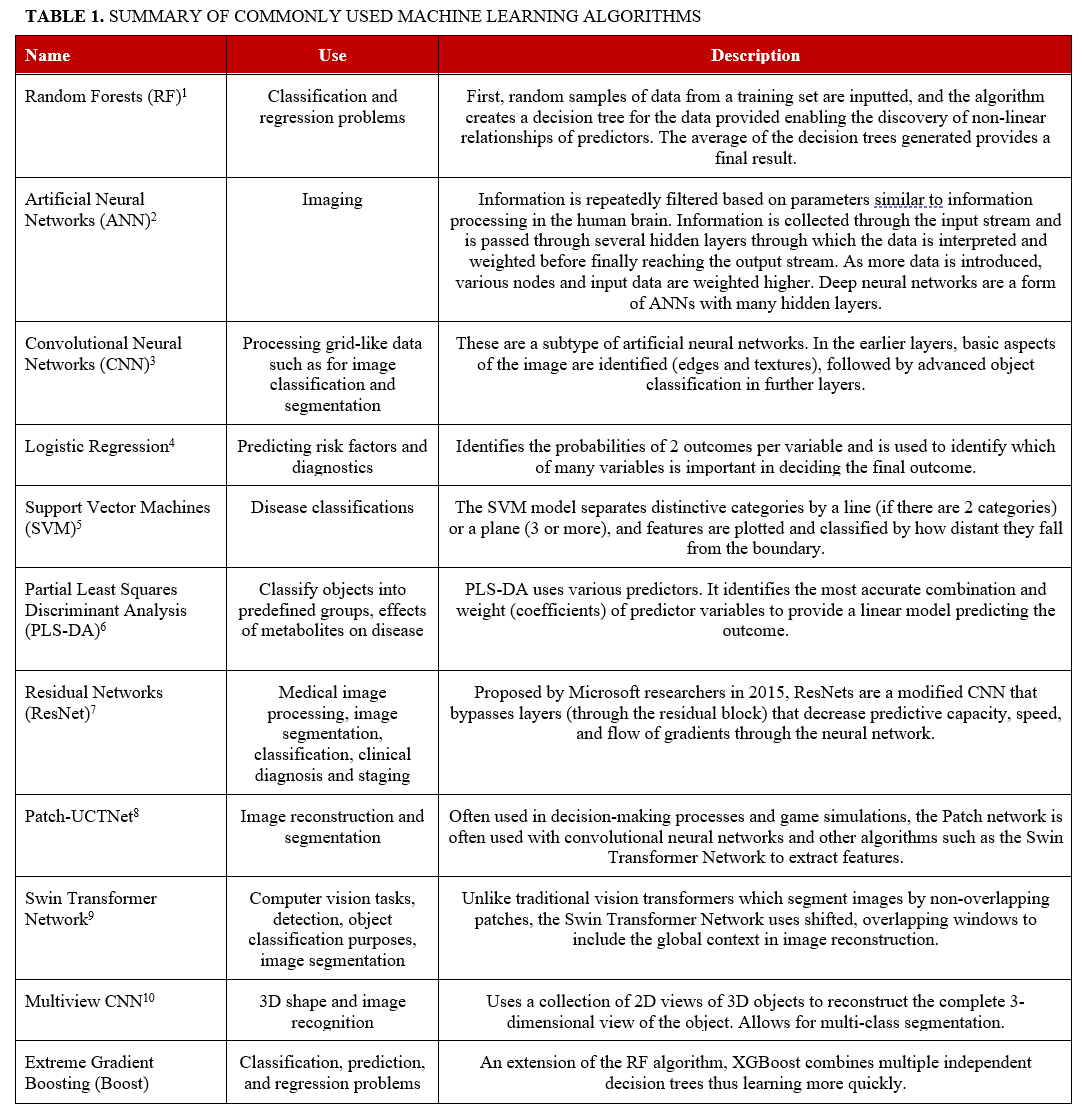
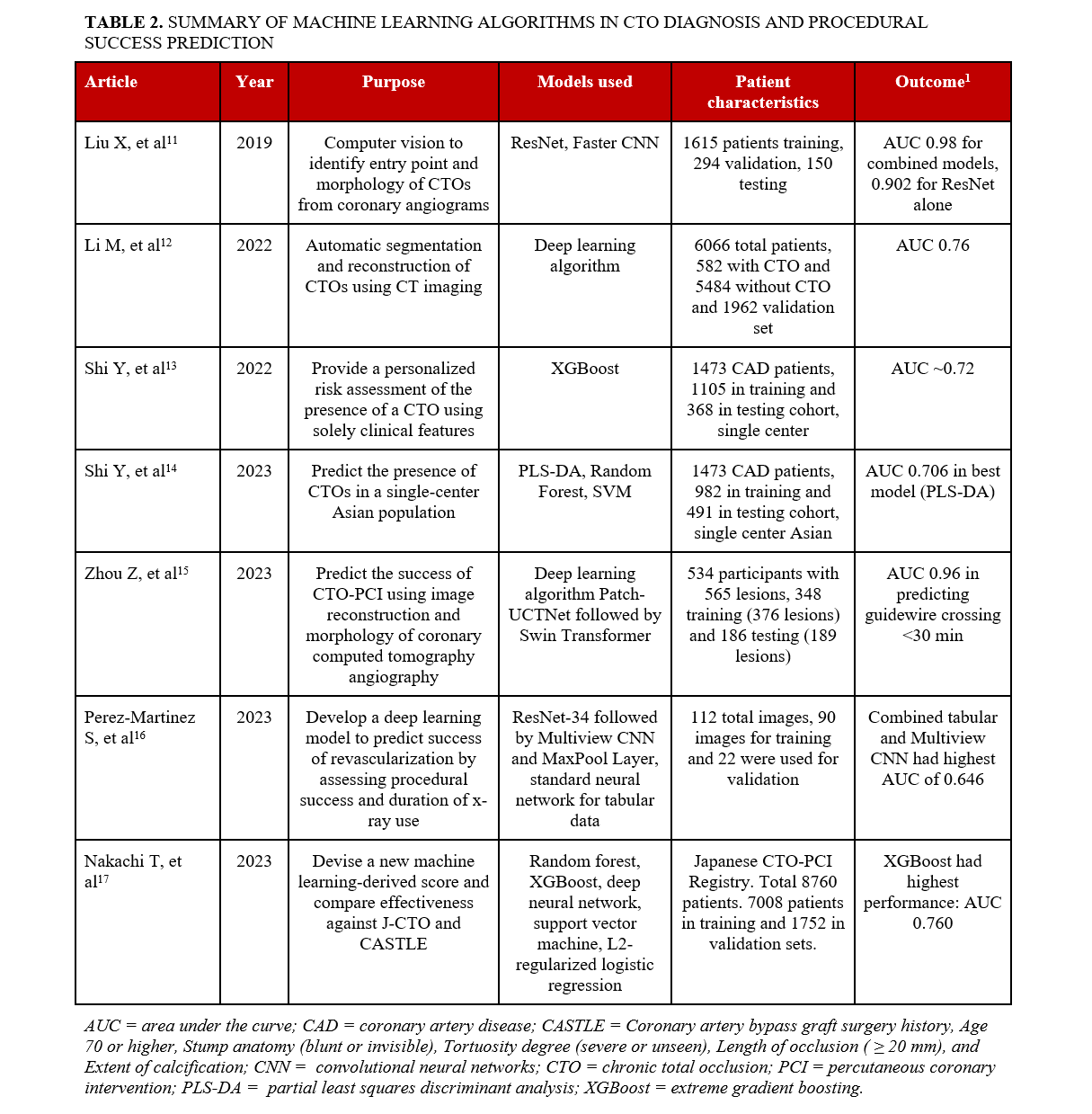
Several machine and deep learning algorithms have been used in many fields of medicine, including cardiology. Such algorithms include Random Forests, Convolutional Neural Networks (CNNs), Artificial Neural Networks (ANNs), Support Vector Machines (SVM), logistic regression, and partial least squares discriminant analysis (PLS-DA) (Table 1).1-10 Numerous models have been developed with the goal of improving preprocedural planning of CTO PCI (Figure, Table 2).11-17
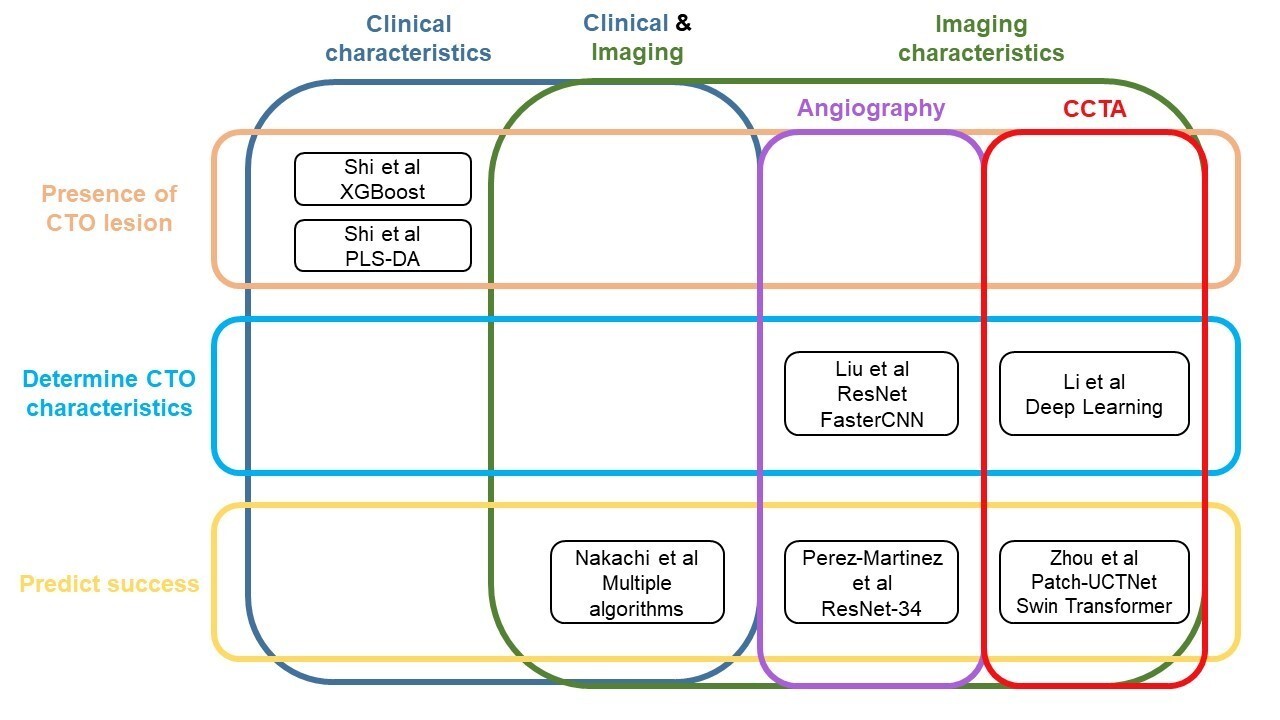
Machine learning for diagnosing the presence of CTOs
Models using clinical characteristics
Shi et al used machine learning algorithms to assess the likelihood of having a CTO using only clinical parameters.13 The study included 1473 patients with coronary artery disease (CAD) from a single center, 1105 in the training cohort and 368 in the testing cohort. A prediction model using extreme gradient boosting (XGBoost) was developed showing that sex, high density lipoprotein, and total cholesterol had the strongest association with the presence of a CTO. The area under the curve (AUC) was 0.724 and 0.719 in the training and testing cohorts, respectively.
Shi et al also used machine learning to identify critical clinical features associated with the presence of coronary CTOs in a single-center Asian patient population. The study included 1473 CAD patients, of whom 317 had a CTO and 1156 did not.14 The data was normalized and log10-transformed, and 3 machine learning algorithms were applied to identify CTO risk factors and presence in patients: PLS-DA, random forest, and support vector machine. The models’ performances were then validated using Monte-Carlo cross-validation, where the data was trained using two-thirds of the sample and validated on one-third. Weights were assigned to clinical factors using Gini impurity, similar to the aforementioned study. The most important variables in the PLS-DA model were B-type natriuretic peptide (BNP), left ventricular end-diastolic diameter, left ventricular ejection fraction, neutrophils, and lymphocyte percentage. The random forest model marked the same variables as the PLS-DA model except for lymphocyte percentage, which was replaced by sex. The support vector machine (SVM) identified prothrombin time, sex, triglyceride glucose (TyG) index, plateletcrit, and BNP (pg/mL) as the most important variables. All 3 models presented sex, neutrophil (NE)%, creatinine, and BNP, indicating significant risk factors in CTO development. The PLS-DA model had an AUC of 0.706, followed by random forest (0.702) and SVM (0.696).
Machine learning for determining CTO characteristics
Models using imaging characteristics
Models using angiography. Liu et al used CNNs to identify the entry point and morphology of CTOs from coronary angiograms.11The algorithm was trained using 1615 cases, validated in 294 cases, and tested on 150 cases, showing an 89.3% accurate detection rate, with 94.5% sensitivity and 89.1% specificity. The study used the entry point of the CTO lesion stump as the object to be detected, and used a residual network (ResNet), a deep learning CNN for computer vision, to detect and classify objects. Subsequently, another algorithm called FasterCNN was used to identify whether the previously detected region truly contained a lesion and to classify it. The proximal CTO cap was classified as blunt (326 cases) and tapered (1732 cases), where a tapered lesion featured a funnel-shaped occluded segment while a blunt one did not. The weights and biases in the pre-trained models were used and applied to the cases for fine-tuning. While applying ResNet alone had an AUC of 0.902, the combined algorithms had an AUC of 0.98, demonstrating outstanding diagnostic accuracy.
Models using CCTA. Li et al used a deep learning algorithm to automatically segment and reconstruct CTOs using computed tomography (CT) imaging.12 It was trained through a retrospective set of 6066 patients, 582 with CTO and 5484 without CTO, and a validation set of 1962 patients, 208 with and 1754 without CTO. The algorithm was expected to reduce both postprocessing and measurement time compared with manual reconstruction. External validation of the model was done using coronary CT angiograms of CTOs from 211 patients with 240 lesions. The algorithm had significantly shorter postprocessing time and excellent correlation with the conventional manual protocol for all quantitative and qualitative CTO parameters evaluated (all correlation coefficients > 0.85; all P < .001). There was no difference in the predictive capacity of the Japan-CTO (J-CTO) score derived from the deep learning algorithm vs the conventional manual protocol (AUC 0.76 vs 0.76; P = .55).
Machine learning for predicting success
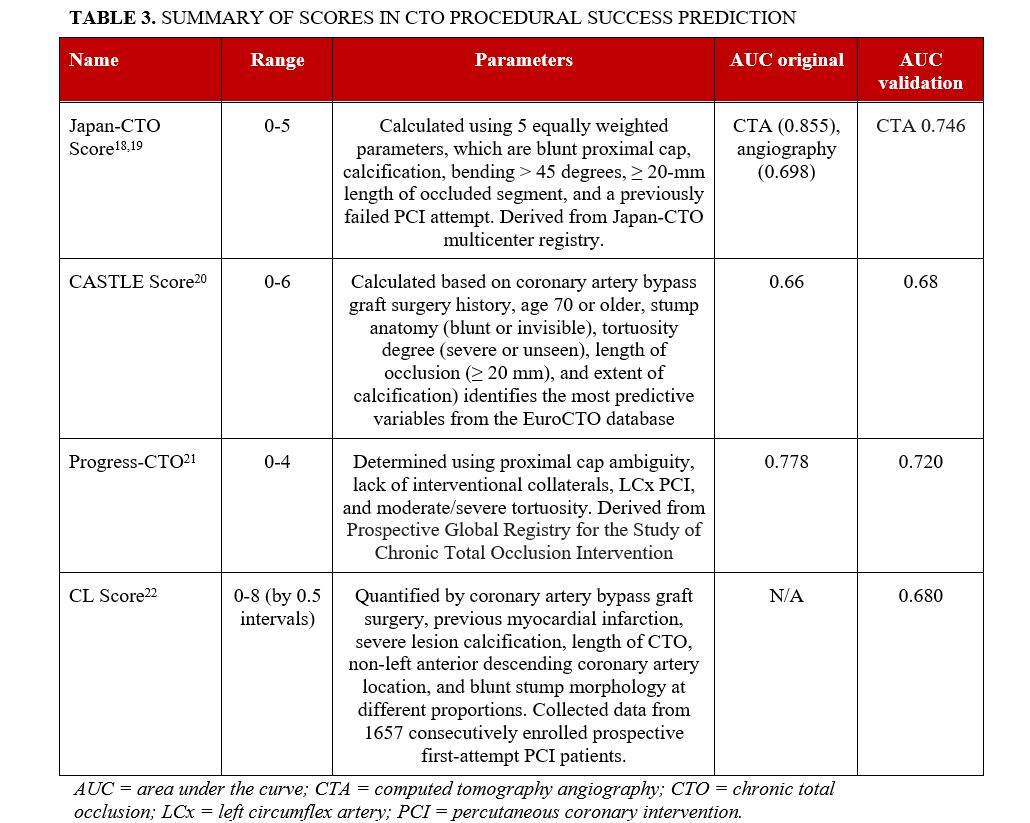
Several clinical scores have been developed to estimate the likelihood of success and procedural efficiency of CTO PCI using traditional statistical methods. The most commonly used score is the J-CTO score, which was developed to predict the likelihood of successful CTO crossing within 30 minutes. The J-CTO score ranges from 0 to 5 and is calculated using 5 equally weighted parameters: blunt proximal cap, calcification, bending greater than 45 degrees, an occlusion length greater or equal to 20 mm, and a previously failed PCI attempt.18,19 The CASTLE score (Coronary artery bypass graft surgery [CABG] history, Age 70 or higher, Stump anatomy [blunt or invisible], Tortuosity degree [severe or unseen], Length of occlusion [20 mm or greater], and Extent of calcification) identifies the most predictive variables from the EuroCTO database.20 The PROGRESS-CTO score (Prospective Global Registry for the Study of Chronic Total Occlusion Intervention) is calculated using proximal cap ambiguity, lack of interventional collaterals, left circumflex (LCX) PCI, and moderate/severe tortuosity.21 The clinical and lesion-related score (CL) takes into account CABG, previous myocardial infarction (MI), severe lesion calcification, length of CTO, non-left anterior descending coronary artery location, and blunt stump morphology at different proportions.22 The AUCs of these scores are modest, as shown in Table 3.
Models using clinical and imaging characteristics
Nakachi et al developed a new machine learning score using clinical metrics and angiographic characteristics for predicting technical success in CTO-PCI and compared its effectiveness with traditional scores such as J-CTO, CL, and CASTLE scores.17 The study included 8760 patients (7990 successful CTO-PCIs) from the Japanese CTO-PCI expert registry featuring 46 operators and prospective, nonrandom enrollment: 80% of the patients were included in the training set and 20% were assigned to the test set. The study included 65 predictor variables in 5 algorithms: random forest, XGBoost, deep neural networks, SVM classifier, and L2-regularized logistic regression. To ensure fair comparison between the algorithms and manual scores, a multivariate logistic regression analysis score (CURRENT) similar to the existing manual scores was developed by assigning points to each strong predictive factor of CTO-PCI failure. The key factors identified were hemodialysis, a CTO vessel diameter of less than 2.5 mm, CTO entry with no stump, severe calcification, lesion bending, a lesion length greater than or equal to 20 mm, proximal right coronary artery disease, and proximal left circumflex artery disease. The XGBoost algorithm exhibited the highest AUC of 0.760, followed by random forest (0.746), deep neural networks (0.737), L2-regularized logistic regression (0.733), and SVM classifier (0.679). The XGBoost also had a higher AUC than the J-CTO score (0.697), CL score (0.662), CASTLE score (0.659), and the newly developed CURRENT score (0.702).
Models using imaging characteristics
Models using angiography. Perez-Martinez et al developed a deep learning model to predict the likelihood of successful revascularization in CTO patients trained with 112 angiography images.16 The outcomes were procedural success and the duration of the time X-ray imaging technology was used. CTO-PCI was successful in 75% of the cases. Images were collected as a single frame selected manually from angiography videos collected between 2011 and 2021 to represent the standard projection of the procedure. Of the total images, 80% were used for training and 20% were used for validation. Of the 80% used for training, 15% were used for validation during the training process. The deep learning algorithm featured several key components. First, a ResNet-34 CNN was used to see if intervention classification could be achieved using a single view. Next, a neural network for tabular data was used to determine its predictive power. A multiview CNN was then used to pass different and complementary views from 4 projections through a feature extraction phase and classify the combined features in a posterior stage. A MaxPool layer was then used to join the features and highlight pertinent information on each view. A ResNet-34 network was then used to create a classification from the information obtained from the 4 views. Finally, a multiview CNN with mixed data including all the views and tabular patient data was developed. The multiview CNN with tabular patient data had the highest AUC of 0.646, followed by the multiview CNN with an AUC of 0.563, single-view CNN with an AUC of 0.343, and tabular data only of 0.292. In comparison, the original J-CTO score had the highest AUC of 0.729. The low-moderate AUC of the deep learning models may be explained by the heterogeneity of the angiography images used and the noise affecting the models’ ability to classify at high precision.
Models using CCTA. Zhou et al used deep learning to predict the success of CTO-PCI using coronary computed tomography angiography (CCTA). The training set included 348 patients (with 376 CTO lesions) prospectively enrolled from 1 tertiary hospital.15 The external test set included 186 patients (with 189 CTO lesions) with CTO lesions from 3 tertiary hospitals with the same inclusion (CAD patients who underwent CCTA before PCI, invasive coronary angiography-confirmed CTO lesions, attempted PCI) and exclusion (history of acute MI within 3 months, CCTA and PCI interval > 1 month, history of CABG and stents, nondiagnostic imaging) criteria as the training set. First, Patch-UCTNet was introduced to reconstruct the 3D structure of the coronary arteries. Next, skeletonization and expansion were utilized to identify the CTO lesions. After that, the Swin Transformer network was applied to extract the CTO features from the CCTA and CTO lesion candidates to generate prediction results of successful CTO-PCI with guidewire crossing under 30 minutes, over 30 minutes, or failed CTO-PCI. The results from the deep learning algorithm were compared against CCTA manual analysis performed by 2 radiologists and against the outcomes of the coronary intervention procedure. The AUC of the deep learning algorithm for predicting successful CTO-PCI with guidewire crossing within 30 minutes in the training and testing sets were 0.97 and 0.96, respectively. The deep learning model also outperformed manual prediction scores in both the success of procedure and guidewire crossing within 30 minutes (P < .05 for both). The sensitivity of the manual prediction (J-CTO) was approximately 47% lower and the accuracy was approximately 22% lower than the deep learning model.
Conclusions
CTO-PCI can be challenging, and the prediction of procedural success is difficult. Machine learning algorithms can serve as a useful tool to diagnose CTOs and aid clinical decision making. Current algorithms can use clinical data as well as imaging to determine CTO anatomic characteristics and predict the likelihood of successful revascularization of CTO lesions. Future research can include the use of systems that analyze CCTA and coronary angiograms directly for planning PCI.
Affiliations and Disclosures
Athanasios Rempakos, MD1; Pranathi Pilla, BSc1; Michaella Alexandrou, MD1; Deniz Mutlu, MD1; Dimitrios Strepkos, MD1; Pedro E.P. Carvalho, MD1; Ozgur Selim Ser, MD1; Ali Bahbah, MD1; Amit Amin, MD2; Anand Prasad, MD3; Lorenzo Azzalini, MD, PhD4; Luiz F. Ybarra, MD5; Olga C. Mastrodemos, BA1; Bavana V. Rangan, BDS, MPH1; Ahmed Al-Ogaili, MD1; Sandeep Jalli, DO1; M. Nicholas Burke, MD1; Yader Sandoval, MD1; Emmanouil S. Brilakis, MD, PhD1
From the 1Minneapolis Heart Institute and Minneapolis Heart Institute Foundation, Abbott Northwestern Hospital, Minneapolis, Minnesota; 2Rush University Medical Center, Chicago, Illinois; 3Department of Medicine, Division of Cardiology, University of Texas (UT) Health San Antonio, San Antonio, Texas; 4University of Washington Medical Center, Washington; 5Western University, London Health Sciences Center, London, Ontario, Canada.
Acknowledgments: The authors are grateful for the philanthropic support of our generous anonymous donors, and the philanthropic support of Drs. Mary Ann and Donald A Sens; Mrs. Diane and Dr. Cline Hickok; Mrs. Wilma and Mr. Dale Johnson; Mrs. Charlotte and Mr. Jerry Golinvaux Family Fund; the Roehl Family Foundation; the Joseph Durda Foundation. The generous gifts of these donors to the Minneapolis Heart Institute Foundation's Science Center for Coronary Artery Disease (CCAD) helped support this research project.
Disclosures: The authors report no financial relationships or conflicts of interest regarding the content herein.
Address for correspondence: Emmanouil S. Brilakis, MD, PhD, Center for Coronary Artery Disease, Minneapolis Heart Institute, 920 E 28th Street #300, Minneapolis, MN 55407, USA. Email: esbrilakis@gmail.com; X: @esbrilakis
References
- Rigatti SJ. Random forest. J Insur Med. 2017;47(1):31-39. doi:10.17849/insm-47-01-31-39.1
- Shahid N, Rappon T, Berta W. Applications of artificial neural networks in health care organizational decision-making: a scoping review. PLoS One. 2019;14(2):e0212356. doi:10.1371/journal.pone.0212356
- Sarvamangala DR, Kulkarni RV. Convolutional neural networks in medical image understanding: a survey. Evol Intell. 2022;15(1):1-22. doi:10.1007/s12065-020-00540-3
- Schober P, Vetter TR. Logistic regression in medical research. Anesth Analg. 2021;132(2):365-366. doi:10.1213/ANE.0000000000005247
- Yu W, Liu T, Valdez R, Gwinn M, Khoury MJ. Application of support vector machine modeling for prediction of common diseases: the case of diabetes and pre-diabetes. BMC Med Inform Decis Mak. 2010;10:16. doi:10.1186/1472-6947-10-16
- Kelly RS, McGeachie MJ, Lee-Sarwar KA, et al. Partial least squares discriminant analysis and Bayesian networks for metabolomic prediction of childhood asthma. Metabolites. 2018;8(4):68. doi:10.3390/metabo8040068
- Xu W, Fu YL, Zhu D. ResNet and its application to medical image processing: research progress and challenges. Comput Methods Programs Biomed. 2023;240:107660. doi:10.1016/j.cmpb.2023.107660
- Song W, Yu H, Wu J. Patch network for medical image segmentation. February 23, 2023. Accessed March 19, 2024. http://arxiv.org/abs/2302.11802
- Liao Z, Fan N, Xu K. Swin Transformer assisted prior attention network for medical image segmentation. Appl Sci. 2022;12(9):4735. doi:10.3390/app12094735
- Seeland M, Mäder P. Multi-view classification with convolutional neural networks. PLoS One. 2021;16(1):e0245230. doi:10.1371/journal.pone.0245230
- Liu X, Du T, Zhang H, Sun C. Detection and classification of chronic total occlusion lesions using deep learning. Annu Int Conf IEEE Eng Med Biol Soc. 2019;2019:828-831. doi:10.1109/EMBC.2019.8856696
- Li M, Ling R, Yu L, et al. Deep learning segmentation and reconstruction for CT of chronic total coronary occlusion. Radiology. 2023;306(3):e221393. doi:10.1148/radiol.221393
- Shi Y, Zheng Z, Liu Y, Wu Y, Wang P, Liu J. Leveraging machine learning techniques to forecast chronic total occlusion before coronary angiography. J Clin Med. 2022;11(23):6993. doi:10.3390/jcm11236993
- Shi Y, Cheng Z, Jian W, Liu Y, Liu J. Machine learning-based analysis of risk factors for chronic total occlusion in an Asian population. J Int Med Res. 2023;51(10):3000605231202141. doi:10.1177/0300060523120214
- Zhou Z, Gao Y, Zhang W, et al. Deep learning-based prediction of percutaneous recanalization in chronic total occlusion using coronary CT angiography. Radiology. 2023;309(2):e231149. doi:10.1148/radiol.231149
- Perez-Martinez S, Fernandez-Cisnal A, Perez-Pelegri M, et al. Deep learning-based predictive model for revascularization of chronic total occlusions on angiographic imaging. Annu Int Conf IEEE Eng Med Biol Soc. 2023;2023:1-4. doi:10.1109/EMBC40787.2023.10340539
- Nakachi T, Yamane M, Kishi K, et al. Machine learning for prediction of technical results of percutaneous coronary intervention for chronic total occlusion. J Clin Med. 2023;12(10):3354. doi:10.3390/jcm12103354
- Fujino A, Otsuji S, Hasegawa K, et al. Accuracy of J-CTO Score derived from computed tomography versus angiography to predict successful percutaneous coronary intervention. JACC Cardiovasc Imaging. 2018;11(2 Pt 1):209-217. doi:10.1016/j.jcmg.2017.01.028
- Christopoulos G, Wyman RM, Alaswad K, et al. Clinical utility of the Japan-chronic total occlusion score in coronary chronic total occlusion interventions: results from a multicenter registry. Circ Cardiovasc Interv. 2015;8(7):e002171. doi:10.1161/CIRCINTERVENTIONS.114.002171
- Szijgyarto Z, Rampat R, Werner GS, et al. Derivation and validation of a chronic total coronary occlusion intervention procedural success score from the 20,000-patient EuroCTO registry: the EuroCTO (CASTLE) score. JACC Cardiovasc Interv. 2019;12(4):335-342. doi:10.1016/j.jcin.2018.11.020
- Christopoulos G, Kandzari DE, Yeh RW, et al. Development and validation of a novel scoring system for predicting technical success of chronic total occlusion percutaneous coronary interventions: the PROGRESS CTO (Prospective Global Registry for the Study of Chronic Total Occlusion Intervention) score. JACC Cardiovasc Interv. 2016;9(1):1-9. doi:10.1016/j.jcin.2015.09.022
- Alessandrino G, Chevalier B, Lefèvre T, et al. A clinical and angiographic scoring system to predict the probability of successful first-attempt percutaneous coronary intervention in patients with total chronic coronary occlusion. JACC Cardiovasc Interv. 2015;8(12):1540-1548. doi:10.1016/j.jcin.2015.07.009