EXPRESS-LAAO Protocol With the New WATCHMAN FLX Pro Device
© 2025 HMP Global. All Rights Reserved.
Any views and opinions expressed are those of the author(s) and/or participants and do not necessarily reflect the views, policy, or position of the Journal of Invasive Cardiology or HMP Global, their employees, and affiliates.
Abstract
This study evaluates the procedural outcomes and short-term follow-up of the WATCHMAN FLX Pro left atrial appendage occlusion (LAAO) device (Boston Scientific) using the EXPRESS-LAAO protocol at a high-volume center. The new device offers enhanced features, including a 40-mm size for large anatomies, additional radiopaque markers for improved positioning, and a hemocompatible coating for faster endothelization. The EXPRESS-LAAO protocol streamlines patient management with pre-procedure computed tomography or cardiac magnetic resonance imaging, intracardiac echocardiography guidance, conscious sedation, and same-day discharge for stable patients. Between June and September 2024, 109 patients (median age 78 years, 48.6% female) underwent LAAO with the WATCHMAN FLX Pro device. Key indications included recurrent falls (33%), gastrointestinal bleeding (29.4%), and very high bleeding risk (21.1%). Procedural success was high (99.1%), with 1 case of cardiac tamponade requiring pericardiocentesis and no in-hospital complications. The 40-mm device was used in 7.3% of cases, proving essential for larger anatomies. At an average follow-up of 49.7 days, there was 1 lacunar stroke without device-related complications and 1 minor bleeding event. The study demonstrates the safety and effectiveness of the WATCHMAN FLX Pro device using the EXPRESS-LAAO protocol. The TruSteer catheter (Boston Scientific) facilitated implantations in complex anatomies, reducing the number of maneuvers. While post-LAAO imaging surveillance was a limitation, this is the first study assessing peri-procedural outcomes with the new device. Findings support its clinical utility in high-risk patients requiring LAAO.
Over the last decade, left atrial appendage occlusion (LAAO) has been established as an alternative treatment for the management of non-valvular atrial fibrillation in patients with high bleeding risk or contraindication to oral anticoagulant therapy
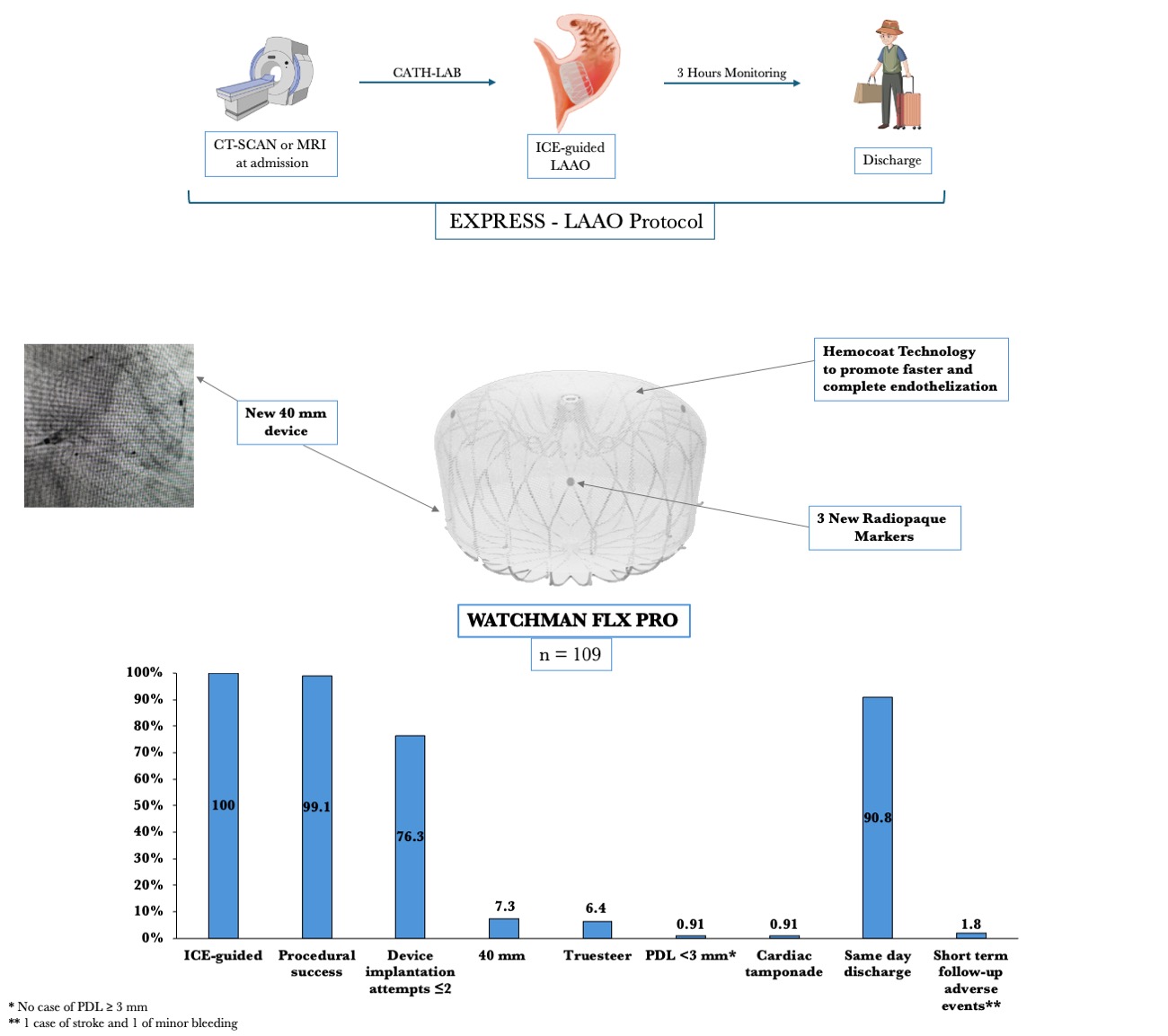
Our hospital is a high-volume center for LAAO and has developed an outpatient treatment protocol (EXPRESS-LAAO protocol). Briefly, patients are admitted on the morning of the procedure and immediately undergo a computed tomography (CT) scan or, if contraindicated, cardiac magnetic resonance imaging (cMRI). In our protocol, transesophageal echocardiography is performed if the patient has an estimated glomerular filtration rate of less than 30 mL/min and an MRI-incompatible device. The radiologic images are reconstructed with the use of TruPlan software (Boston Scientific) to rule out left atrial appendage (LAA) thrombus and to provide additional data for the procedure, such as transeptal planning, automated device sizing, and compression based on the LAA appendage area-derived diameter. The procedure is then performed under conscious sedation and guided by intracardiac echocardiography (ICE). At the end of the procedure, the patient is monitored for 3 hours and then discharged if clinically stable and if there are no signs of bleeding or new pericardial effusion.
This study aims to assess the procedural outcomes and short-term clinical and imaging surveillance (CT-scan) at 4 months of the first consecutive, prospective, real-world enrolled patients who received the new WATCHMAN FLX Pro device at our institution using the EXPRESS-LAAO protocol.
The protocol was approved by the Institutional Review Board and all patients provided informed consent for the procedure. All endpoints were defined according to the Munich Consensus and Position Statement on Cardiac Computed Tomography Following LAAO for the definitions of device-related thrombus (DRT) and peri-device leak (PDL).4,5 Clinical follow-up data were obtained by a clinical visit 30 to 60 days after the procedure or review of medical records. Continuous variables were expressed as the mean ± SD or the median and IQR as appropriate, and categoric variables were expressed as a percentage.
From June to September 2024, 109 consecutive patients underwent LAAO with the WATCHMAN FLX PRO device and were prospectively enrolled in our study. The median age was 78 years, and 48.6% of the patients were female. Median BMI was 27.4 kg/m2, hypertension affected most of the subjects (92.7%), and 24.8% had diabetes. The average left ventricle ejection fraction was 51%. The main indications for LAAO were recurrent falls (33%), gastrointestinal bleeding (29.4%), very high bleeding risk (HAS-BLED ≥ 4, 21.1%), and blood cell dyscrasia (17.4%). Pre-procedural imaging was performed with CT scan (96.3%) or cMRI (3.7%). The median LAA ostium area and LAA ostium area-derived diameter were 5.45 cm2 and 24.50 mm, respectively. All procedures were ICE-guided under conscious sedation using intravenous fentanyl and midazolam. The most used WATCHMAN Access System was the FXD Double Curve (93.7%) (Boston Scientific), followed by the new steerable TruSteer catheter (Boston Scientific) in cases of anterior chicken wing LAA morphology. The total number of devices used was 110 (the size for 1 patient was changed). The new 40-mm device was implanted in 8 (7.3%) patients, including 3 patients who had been previously excluded (1 patient with a large LAA ostia and 2 patients from aborted procedures after wide peri-device leak with the 35-mm device. Procedural success was high (99.1%), with 1 case of cardiac tamponade requiring pericardiocentesis. No post-procedural or in-hospital complications have been reported. A total of 99 (90.8%) patients were discharged on the same day, while the remaining subjects were hospitalized for social or other medical reasons. At 49.7 average days of follow-up, there was 1 case of lacunar stroke without device-related thrombosis or leak, and 1 case of minor bleeding. At 4 months, CT-scan follow-up was available in 95 (87.1%) patients, reporting 4 (4.2%) and 10 (10.5%) cases of DRT and PDL, respectively (Table).
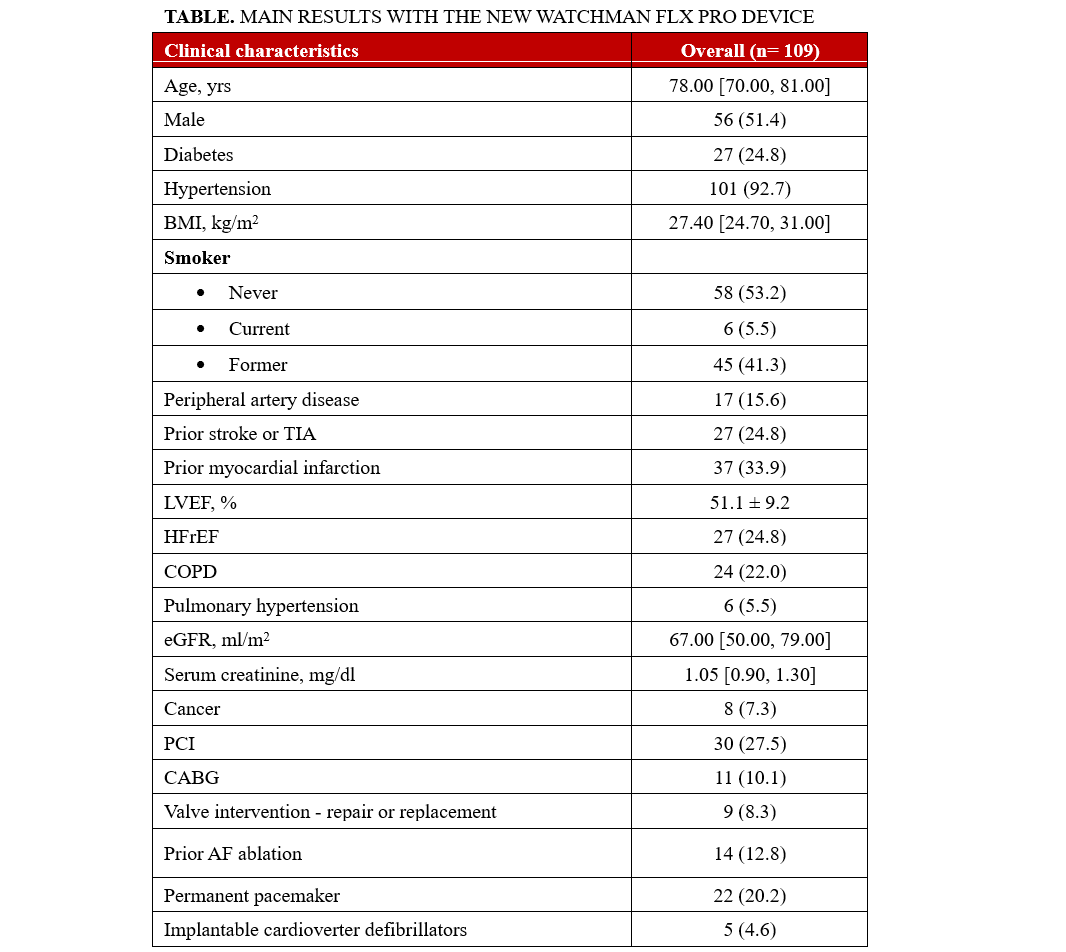
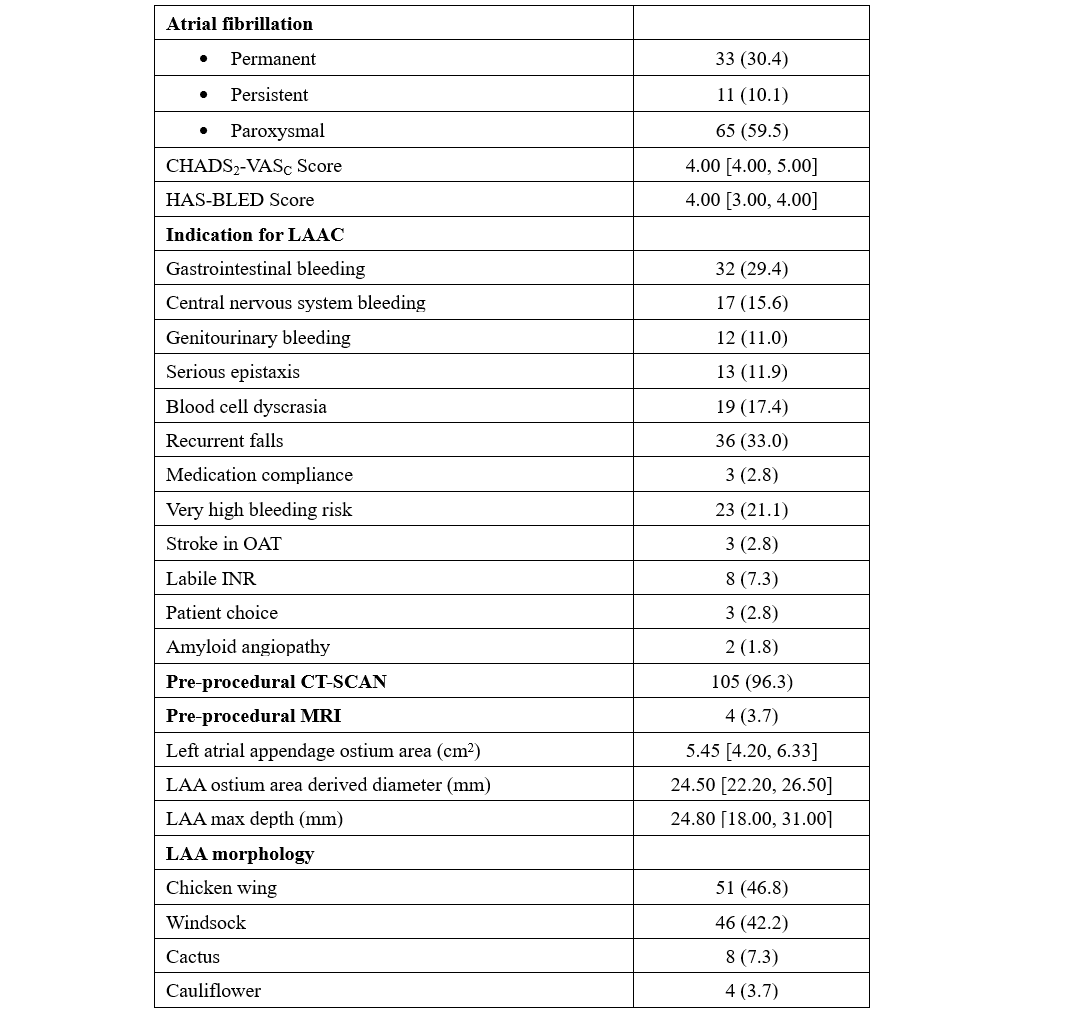
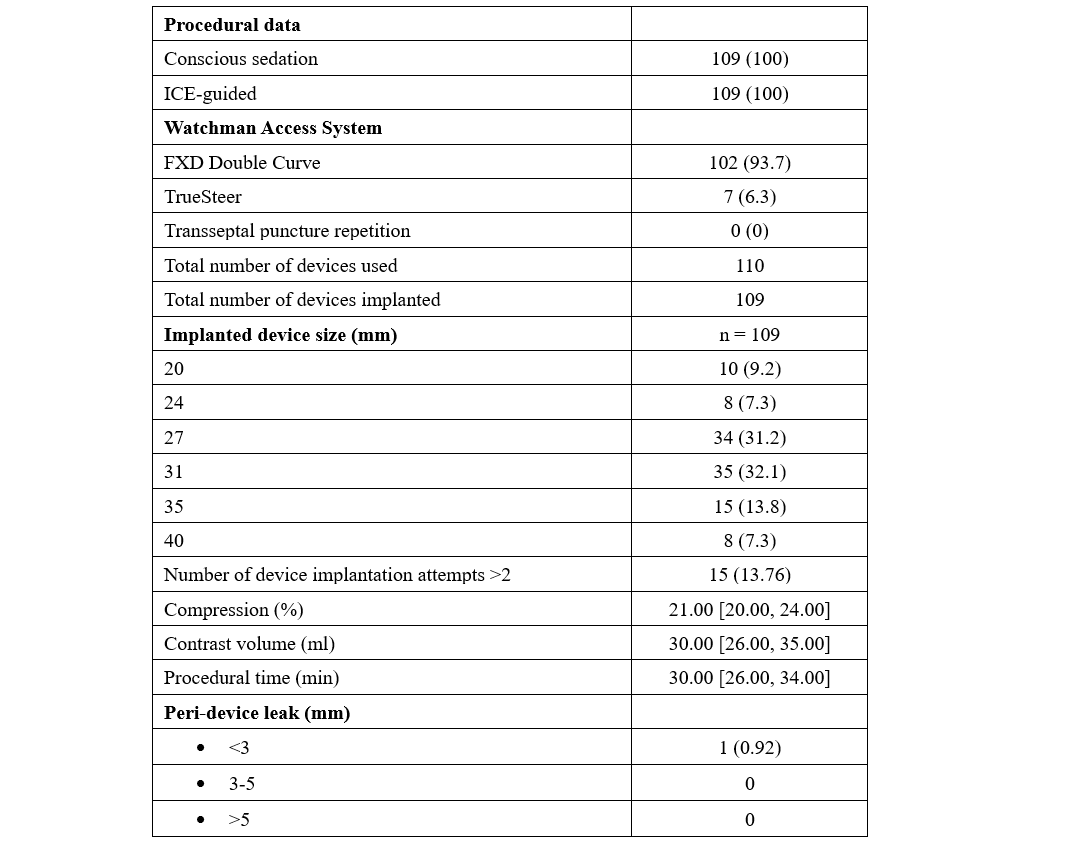
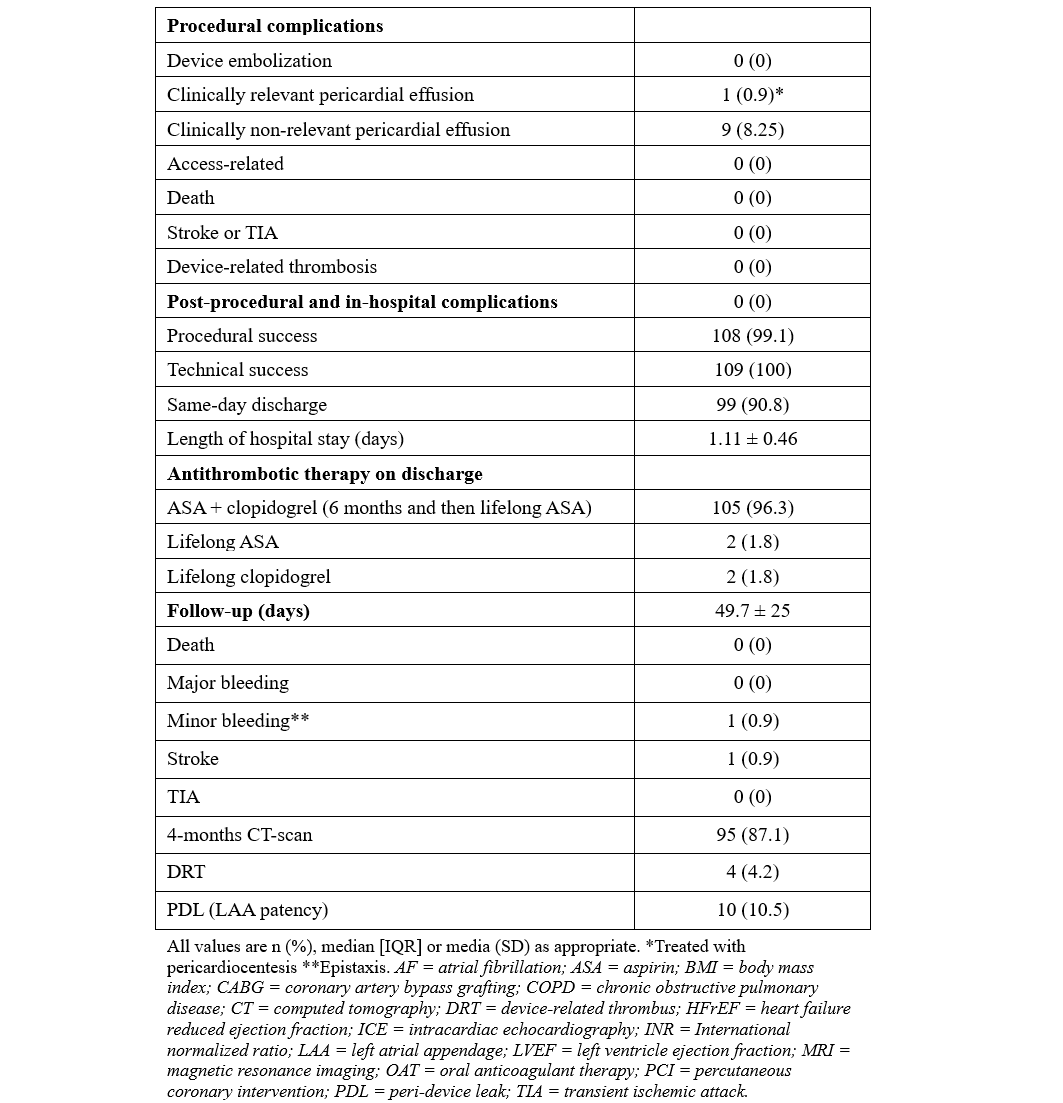
The results of our study highlight the safety and effectiveness of the WATCHMAN FLX Pro device using the EXPRESS-LAAO protocol. The 40-mm device was successfully implanted in 7.4% of the cases, underling the necessity of this large device in selective cases. The TruSteer steerable catheter was helpful in complex anatomies, reducing the number of maneuvers in the LAA to achieve ostium-catheter coaxiality. Additionally, the low rate of peri-procedural events and the satisfying results in terms of DRT and PDL at the 4-month follow-up using our EXPRESS-LAAO protocol are reassuring. To the best of our knowledge, this is the first study to describe the safety and the efficacy of the new WATCHMAN FLX Pro device and TruSteer catheter; larger studies with long-term clinical follow-up are awaited.
Affiliations and Disclosures
Marco Frazzetto, MD, MSc1; Luis Augusto Palma Dallan, MD, PhD1; Pedro Soares Texeira, MD1; Philippe Taieb, MD1; Julia Tirelli, MD1; Rafey Feroze, MD1; Nawaf Alhabdan, MD2; Alexander Cove, MD2; Yusef Saeed, MD2; Anene Ukaigwe, MD1; Guilherme Attizzani, MD1; Steven J. Filby, MD1
From the 1Division of Cardiology, Harrington Heart & Vascular Institute, University Hospitals Cleveland Medical Center, Cleveland, Ohio; 2Department of Medicine, University Hospitals, Cleveland Medical Center, Cleveland, Ohio.
Acknowledgments: The authors are grateful to all nurses, technicians, and valve clinic coordinators who care for our patients.
Disclosures: Dr Ukaigwe is a consultant for Medtronic. Dr Attizzani is a consultant for, serves as a proctor for, is on the advisory board of, and receives research grants from Medtronic, Dasi Simulations, Boston Scientific, and Abbott Vascular. Dr Filby is a consultant for Boston Scientific. The remaining authors report no financial relationships or conflicts of interest regarding the content herein.
Address for correspondence: Steven J. Filby, MD, Division of Cardiology, Harrington Heart & Vascular Institute, University Hospitals Cleveland Medical Center, Cleveland, OH 44106, USA. Email: steven.filby@uhhospitals.org; X: @FrazzettoMarco
References
1. Reddy VY, Sievert H, Halperin J, et al; PROTECT AF Steering Committee and Investigators. Percutaneous left atrial appendage closure vs warfarin for atrial fibrillation: a randomized clinical trial. JAMA. 2014;312(19):1988-1998. doi:10.1001/jama.2014.15192
2. Wass SY, Galo J, Yoon S-H, et al. Predictors of successful same-day discharge and 1-year outcomes after left atrial appendage closure. Catheter Cardiovasc Interv. 2022;100(7):1307-1313. doi:10.1002/ccd.30464
3. Sanfilippo C, Frazzetto M, Costa G, et al. Safety and efficacy of percutaneous left atrial appendage closure without preprocedural imaging. Circ Cardiovasc Interv. 2024;17(7):e014183. doi:10.1161/CIRCINTERVENTIONS.124.014183
4. Tzikas A, Holmes DR Jr, Gafoor S, et al. Percutaneous left atrial appendage occlusion: the Munich consensus document on definitions, endpoints, and data collection requirements for clinical studies. Europace. 2017;19(1):4-15. doi:10.1093/europace/euw141
5. Korsholm K, Iriart X, Saw J, et al. Position statement on cardiac computed tomography following left atrial appendage occlusion. JACC Cardiovasc Interv. 2024;17(15):1747-1764.












