Boston Scientific Obtains CE Mark for FARAPOINT™ Pulsed Field Ablation Catheter
FARAPOINT PFA Catheter delivers effective, safe and consistent ablation for right atrial flutter
FARAPOINT PFA Catheter delivers effective, safe and consistent ablation for right atrial flutter
Boston Scientific Press Release
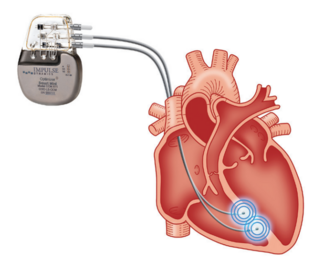
PARIS, France, 27 November, 2025 – Boston Scientific Corporation (NYSE: BSX) today announced it has received CE mark for the FARAPOINT™ Pulsed Field Ablation (PFA) Catheter, the newest PFA catheter in the company’s electrophysiology portfolio. The FARAPOINT PFA Catheter is used for the treatment of right atrial flutter (AFL), delivering ablation to the cavotricuspid isthmus (CTI) area of the heart. The CE mark represents a new cardiac arrhythmia indication for the FARAPULSE™ PFA Platform – the most clinically proven PFA system – and brings the potential benefits of the procedure to those living with AFL.
AFL typically presents with a consistent yet rapid heart rhythm, whereas atrial fibrillation (AF) is marked by a disorganised, irregular and typically fast heart rhythm. AF can be classed as persistent, where episodes last for more than seven days, or paroxysmal, characterized by intermittent episodes. A person can have both AFL and AF and may experience shortness of breath, chest tightness, and fatigue. AF and AFL represent a significant public health challenge in Europe, with the number of cases projected to rise over the next 20 years. In particular, the incidence and prevalence of AF are increasing rapidly.1
The FARAPOINT PFA Catheter provides CTI ablation for right atrial flutter, delivering predictable, point-by-point linear and focal lesions across complex heart anatomies and through scar tissue. The catheter performs optimised and controlled lesions, with a depth of up to 7mm, ensuring effective conduction block without compromising the safety of the surrounding cardiac tissue.
“The FARAPULSE PFA Platform is a transformational advancement in the treatment of paroxysmal and persistent atrial fibrillation, and the most clinically validated PFA system, with 500,000 patients treated worldwide,” said Caroline Bravo, vice president, Rhythm Management EMEA, Boston Scientific. “Today’s announcement reflects the strength of the clinical evidence demonstrating FARAPULSE’s safety, effectiveness and reliability for people living with atrial flutter.”
The CE mark submission was supported by data from Phase II of the ADVANTAGE AF clinical trial, demonstrating the efficacy and safety of the FARAPULSE PFA Platform and the FARAPOINT PFA Catheter as an adjunctive treatment for persistent AF and AFL. The study found that 97.9% of the 141 patients who received CTI ablation with the FARAPOINT PFA Catheter did not experience AFL recurrence, a comparable efficacy rate to the 98% seen with radiofrequency ablation (RFA), the standard of care, studied in Phase I of the trial. Similarly, CTI ablation with the FARAPOINT PFA Catheter saw comparable safety rates to RFA (2.1% versus 2.0% at 90 days following the procedure), and the administration of prophylactic nitro-glycerine during CTI ablation led to zero coronary artery spasms.
A sub-analysis showed that the FARAPOINT PFA Catheter provided significantly greater predictability in CTI applications and overall procedure times compared with RFA. These findings point to real-world procedural reliability, potentially resulting in more consistent workflows and procedural efficiencies.
“Pulsed field ablation is already proven to deliver more effective ablation procedures for the treatment of atrial fibrillation, compared with standard radiofrequency ablation and cryoablation treatments,” said Andrea Natale, professor of medicine, Cardiology Division, University of Rome Tor Vergata, Italy. “We are now seeing the promise of PFA in treating atrial flutter, and the FARAPOINT PFA Catheter, along with wider mapping and 3D visualization abilities of the FARAPULSE PFA Platform, can offer physicians the ability to optimise workflows and tailor atrial flutter procedures.”
Boston Scientific will launch the FARAPOINT PFA Catheter in the EMEA region. More information is available here.
About Boston Scientific
Boston Scientific transforms lives through innovative medical technologies that improve the health of patients around the world. As a global medical technology leader for more than 45 years, we advance science for life by providing a broad range of high-performance solutions that address unmet patient needs and reduce the cost of healthcare. Our portfolio of devices and therapies helps physicians diagnose and treat complex cardiovascular, respiratory, digestive, oncological, neurological and urological diseases and conditions. Learn more at www.bostonscientific.eu and connect on LinkedIn.
1 Xie M, et al. The burden of atrial fibrillation/atrial flutter in Europe from 1990 to 2021, with a forecast of incidence through 2044. Front Cardiovasc Med. 2025 Jun 18;12:1606024.