Intracardiac Echocardiography Guided Pulsed Field Ablation and Left Atrial Appendage Closure: A One-Stop Solution for Rhythm and Stroke Management
© 2026 HMP Global. All Rights Reserved.
Any views and opinions expressed are those of the author(s) and/or participants and do not necessarily reflect the views, policy, or position of EP Lab Digest or HMP Global, their employees, and affiliates.
EP LAB DIGEST. 2026;26(2):12-14.
Yoel R Vivas, MD, FHRS,1 and Abhi S Grewal2
1The Arrhythmia Center of South Florida, Delray Beach, Florida; 2Lake Erie College of Osteopathic Medicine, Greensburg, Pennsylvania
Recent advancements in cardiac electrophysiology have brought to light compelling evidence regarding the efficacy and safety of left atrial appendage closure (LAAC) in conjunction with catheter-based atrial fibrillation (AF) ablation, while significantly reducing the risk of major or clinically relevant bleeding during long-term follow-up.1 This combined approach, particularly when guided by intracardiac echocardiography (ICE), presents a promising strategy for managing complex cases of AF.2
Moreover, the integration of ICE facilitates real-time visualization and guidance during both pulsed field ablation (PFA) and LAA occlusion (LAAO), thereby enhancing procedural safety and efficacy while potentially obviating the need for transesophageal echocardiography (TEE) and general anesthesia.3 This report details a case illustrating the successful concomitant use of PFA for pulmonary vein (PV) and posterior wall isolation and LAAO in a single procedure, guided entirely by ICE, highlighting its potential as a “one-stop” solution for complex AF management.4,5
Case Presentation
A 73-year-old woman with symptomatic persistent AF (CHA₂DS₂-VASc 5, HAS-BLED 3) presented for rhythm and stroke risk management. Her initial extended monitor showed prolonged episodes of rapid ventricular response. Her left ventricular ejection fraction (LVEF) had deteriorated from 60% to 45%. Additional medical history was significant for protracted gastrointestinal bleeding from an unknown source, with secondary symptomatic anemia, and poor tolerance to dual oral anticoagulation (OAC). Given her high risk of both thromboembolism and bleeding, a combined procedure incorporating pulmonary vein isolation (PVI) with PFA and LAAC was performed to address rhythm control and stroke prevention simultaneously.
Procedure
Vascular access was obtained via ultrasound-guided Seldinger technique from the right and left femoral veins. A multipolar electrode catheter was positioned with the help of the mapping system in the right atrium (RA), crista terminalis, and coronary sinus (Livewire Duo-Decapolar, Abbott). A transseptal puncture was performed with a deflectable sheath (Faradrive Steerable Sheath, Farapulse PFA System, Boston Scientific) and confirmed by a leftward jump into the LA. The LA pressure was 17 mmHg. Heparin was administered to maintain an activated clotting time (ACT) above 350 seconds. The Faradrive sheath was retracted to the RA, keeping the pigtail wire in the left upper superior PV. The ICE probe (ViewFlex, Abbott) was then advanced to the LA for direct visualization of the pentaspline ablation catheter.
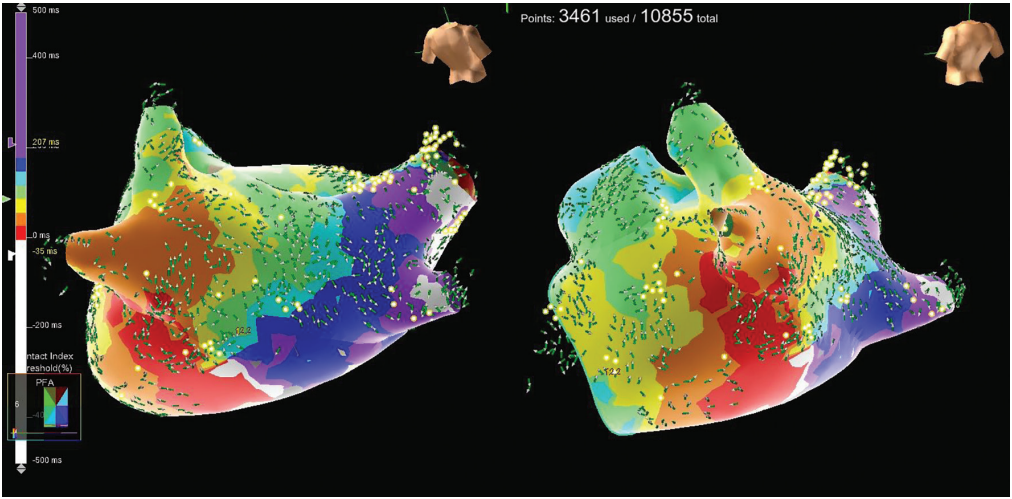
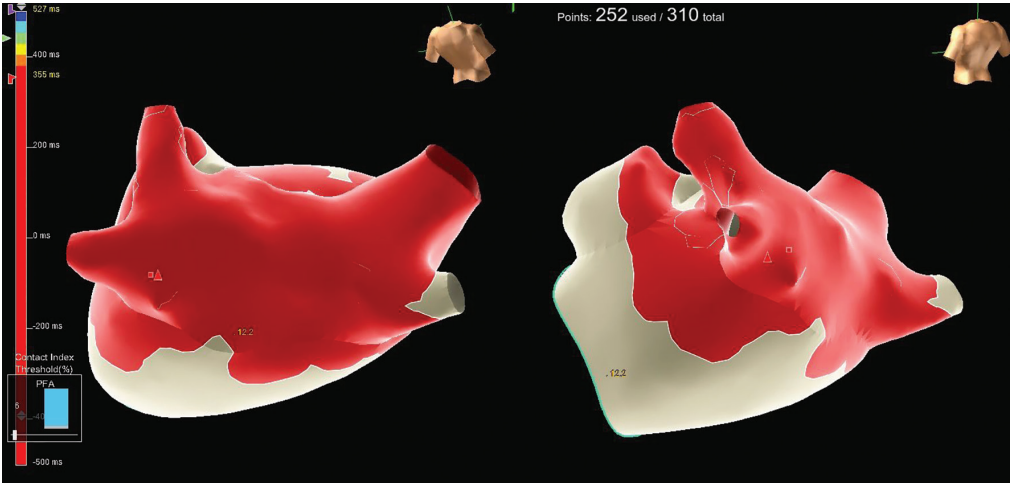
The patient was cardioverted to sinus rhythm with 200 J biphasic energy. A 3-dimensional (3D) electroanatomic map of the LA was created using the EnSite system (Abbott) with a high-resolution Advisor HD Grid Mapping Catheter (Abbott). A high-resolution map of the PVs, LA body, and LAA was obtained (Figure 1). One milligram of atropine was given prior to first delivery of pulsed field. A total of 40 applications were delivered to achieve PV antral and posterior wall isolation, with rapid and clean electrogram attenuation confirming isolation (Figure 2, Video 1). Exit block was confirmed using high-output pacing with the Farawave catheter in the “olive” configuration, and adenosine was given to uncover dormant conduction at the end of the case.
Following ablation, the Faradrive sheath was exchanged for a TorqVue Delivery System (Abbott). The ICE probe was moved to a supramitral view and full measurements of the LAA were obtained. Based on the complexity, shape, and size of the ostium, a dual-seal mechanism LAA device was selected. ICE imaging was used to guide device placement and positioning, confirming that two-thirds of the device lobe were distal to the left circumflex coronary artery, with appropriate compression and coaxial alignment within the LAA. The disc and lobe of the occlusion device were separated, with the elliptical shape of the disc visualized, thus ensuring placement in accordance with the CLOSE criteria.6 Final angiography confirmed full closure without peri-device leakage (Video 2).
The delivery system and ICE catheter were withdrawn from the LA, and the ACT was reversed with protamine sulfate. Vascular closure was achieved using the Perclose system (Abbott). Total operative fluoroscopy was noted at 2.0 minutes. The patient left the laboratory in stable cardiopulmonary condition.
Results and Efficiency
Throughout the case, ICE provided continuous visualization of catheter manipulation and contact quality, potentially preventing complications such as perforation or device migration. The combination of ICE and 3D electroanatomical mapping enabled near-zero fluoroscopy navigation and faster transseptal-to-closure workflow. No complications occurred.
Post-procedure TEE at 6 weeks confirmed complete LAAO without leak or thrombus formation. The patient remained in sinus rhythm on follow-up with no thromboembolic or bleeding events.
Discussion
This case demonstrates the capability of ICE to provide direct, real-time intracardiac imaging without obstruction, facilitating concomitant ablation and occlusion procedures. In contrast, TEE, although offering excellent image quality, is more invasive and typically requires sedation or general anesthesia, thereby increasing procedural complexity and recovery time.7 The tissue selectivity of PFA further simplifies the ablation component, minimizing the risk of collateral injury to surrounding structures. In fluoroscopy-guided procedures, mean fluoroscopic time is shown at 15.90 ± 5.17.8 The usage of ICE enables the mitigation of fluoroscopy and allows for a streamlined operating room.
Lyan et al9 conducted a retrospective analysis of 481 patients who underwent radiofrequency ablation for PVI. The study demonstrated equivalent 12-month symptom-free intervals between ICE-guided and fluoroscopy-guided groups. However, ICE provides real-time visualization that may enhance procedural safety while maintaining the established efficacy of fluoroscopy-guided ablation. Exposure to fluoroscopy in invasive electrophysiology procedures has been shown to increase total lifetime risk of fatal malignancy, with the carcinogenic effect of ionizing radiation multiplying in a dose-dependent fashion.10 This advancement highlights the potential for a completely TEE-free approach in complex arrhythmia interventions, with minimal radiation exposure for both patients and medical staff.
The OPTION trial specifically investigated the efficacy and safety of LAAO combined with catheter ablation in a single procedure, further exploring strategies to improve symptom control and stroke prevention.1 It elucidated the long-term clinical outcomes associated with different procedural sequences, with the trial specifically comparing catheter ablation with OAC versus patients undergoing simultaneous LAAO and catheter ablation, showing safety in the combined approach, with a lower risk of major hemorrhagic event.11 In a meta-analysis by Serpa et al,12 comparison of ICE and fluoroscopy in LAAO procedures revealed a modest increase in procedural success as the primary endpoint, with similar complication rates, but did note an increase in incidence of pericardial effusion in the ICE group, which remains an avenue to be further explored. The overall advantages of ICE-guided LAAO are evident, but further research would help delineate the approach in future endeavors.
With increasing operator experience and advances in multimodality imaging, the concomitant approach to AF ablation and thromboembolic event prevention with LAAO is a practical, efficient, and patient-centered strategy. The EMERGE LAA Postapproval Study by Ellis et al13 further supports the safety and feasibility of performing concomitant catheter ablation and LAAO using the AMPLATZER Amulet LAA occluder (Abbott) in high-risk patients with AF. For appropriately selected patients with symptomatic AF and intolerance to OAC, this integrated workflow provides rhythm control, stroke protection, and a reduced procedural burden within a single visit—a one-stop solution.
Funding: No internal or external funding was used in this manuscript. Sincerest gratitude to Juan Navarrette (Abbott) for images.
Disclosures: The authors have completed and returned the ICMJE Form for Disclosure of Potential Conflicts of Interest. Abhi S Grewal reports no disclosures. Dr Vivas, reports consulting fees for Abbott and assists in training for Boston Scientific and Abbott.
Video 1
Video 1. ICE sequence demonstrating isolation of the right superior pulmonary vein (RSPV). The images highlight the spatial relationship between the pulmonary artery and ablation catheter, showing maintained coaxial alignment throughout energy delivery to ensure optimal contact, stability, and effective lesion formation during RSPV isolation. Video available at www.eplabdigest.com.
Video 2
Video 2. ICE sequence demonstrating deployment of a double-seal occlusion device. The device is transitioned from a ball configuration, allowing rotational movement, to a triangular shape through forward pressure applied by the operator for safe advancement, and finally to a lobe configuration for anchoring within the LAA. It is followed by controlled unsheathing of the disc under real-time ICE guidance, confirming stable positioning and complete seal within the target structure. Video available at www.eplabdigest.com.
References
- Wazni OM, Saliba WI, Nair DG, et al. Left atrial appendage closure after ablation for atrial fibrillation. N Engl J Med. 2025;392(13):1277-1287. doi:10.1056/nejmoa2408308
- Díaz JC, Duque M, Marín JE, et al. Intracardiac echocardiography-guided left atrial appendage occlusion. Arrhythm Electrophysiol Rev. 2024:13:e03. doi:10.15420/aer.2023.29
- Patel A, Venkataraman R, Schürmann P, Dave AS, Valderrábano M. Left atrial appendage occlusion using intracardiac echocardiography. Heart Rhythm. 2021;18(2):313-317. doi:10.1016/j.hrthm.2020.09.021
- Bianchini L, Moltrasio M, Fassini G, et al. Pulsed-field ablation of pulmonary vein and left atrial posterior wall combined with left atrial appendage occlusion as a single procedure. Pacing Clin Electrophysiol. 2024;47(5):691-693. doi:10.1111/pace.14823
- Shang X, Sun M, Wang Z, Jin Z, Liang M. Comparison of intracardiac vs. transesophageal echocardiography for “one-stop” procedures of combined radiofrequency catheter ablation and left atrial appendage closure with the Watchman device in the treatment of atrial fibrillation. Front Cardiovasc Med. 2023:10:1265550. doi:10.3389/fcvm.2023.1265550
- Singleton MJ, Osorio J. Pulsed field ablation for atrial fibrillation: now available without fluoroscopy. J Interv Card Electrophysiol. 2024 Dec 4. doi:10.1007/s10840-024-01933-5
- Ellis CR. Amplatzer Amulet left atrial appendage occluder: a step-by-step guide to device implantation. J Cardiovasc Electrophysiol. 2022;33(8):1881–1887. doi:10.1111/jce.15420.
- Varughese VJ, Pollock JD, Richardson C, et al. Intracardiac echo versus fluoroscopic guidance for pulsed field ablation: single-center real-life study. Biomedicines. 2025;13(5):1186. doi:10.3390/biomedicines13051186
- Lyan E, Tsyganov A, Abdrahmanov A, et al. Nonfluoroscopic catheter ablation of paroxysmal atrial fibrillation. Pacing Clin Electrophysiol. 2018;41(6):611-619. doi:10.1111/pace.13321
- Picano E, Piccaluga E, Padovani R, et al. Risks related to fluoroscopy radiation associated with electrophysiology procedures. J Atr Fibrillation. 2014;7(2):1044. doi:10.4022/jafib.1044.
- Phillips K, Romanov A, Artemenko S, et al. Combining left atrial appendage closure and catheter ablation for atrial fibrillation: 2-year outcomes from a multinational registry. Europace. 2020;22(2):225-231. doi:10.1093/europace/euz286
- Serpa F, Rivera A, Fernandes JM, et al. Intracardiac vs transesophageal echocardiography for left atrial appendage occlusion: an updated systematic review and meta-analysis. Heart Rhythm. 2025;22(3):786-795. doi:10.1016/j.hrthm.2024.08.027
- Ellis CR, Lakkireddy D, Makkar A, et al. Concomitant catheter ablation and left atrial appendage occlusion with the Amulet occluder from EMERGE LAA post-approval study. JACC Clin Electrophysiol. 2025;11(8):1869–1873. doi:10.1016/j.jacep.2025.05.002.











