The SELUTION4ISR Clinical Trial
© 2026 HMP Global. All Rights Reserved.
Any views and opinions expressed are those of the author(s) and/or participants and do not necessarily reflect the views, policy, or position of Cath Lab Digest or HMP Global, their employees, and affiliates.
Donald E. Cutlip, MD
Department of Medicine, Division of Cardiology, Beth Israel Deaconess Medical Center; Chief Medical Officer, Baim Institute for Clinical Research Boston, Massachusetts
Disclosures: Dr. Cutlip reports grant/research support from Cordis (Med Alliance) and Corvia Medical, and consultant fees/honoraria from Boston Scientific.
Dr. Cutlip can be contacted at dcutlip@bidmc.harvard.edu.
Click here for a PDF of this article, courtesy of Cath Lab Digest.
SELUTION4ISR is a prospective, international, randomized, single-blind clinical trial evaluating a sirolimus-eluting balloon for the treatment of coronary in-stent restenosis in arteries with up to two layers of bare-metal or drug-eluting stents (NCT04280029).
A total of 418 patients were randomized to either the sirolimus-eluting balloon group (SELUTION Sustained Limus Release [SLR], Cordis) (n=210) or blended standard of care control group (n=208, with 165 [80%] receiving a drug-eluting stent and [20%] 43 patients receiving plain balloon angioplasty). The primary endpoint is target lesion failure, consisting of cardiac death, target vessel myocardial infarction, and clinically driven target lesion revascularization.
Dr. Cutlip presented SELUTION4ISR one-year results on behalf of the trial investigators at the October TCT 2025 meeting in San Francisco, California.1 The study was funded by M.A. Med Alliance SA (a Cordis Company).
What was the goal of SELUTION4ISR?
 The main goal was to conduct a trial that would allow us to demonstrate the safety and effectiveness of the Selution drug-eluting balloon. At the time the trial was designed, there was no drug-eluting balloon approved in the United States, so we needed to go through the FDA approval pathway.* That was really the primary objective of the trial, to show that the device performed safely and effectively in patients with in-stent restenosis (ISR).
The main goal was to conduct a trial that would allow us to demonstrate the safety and effectiveness of the Selution drug-eluting balloon. At the time the trial was designed, there was no drug-eluting balloon approved in the United States, so we needed to go through the FDA approval pathway.* That was really the primary objective of the trial, to show that the device performed safely and effectively in patients with in-stent restenosis (ISR).
How did the Selution drug-eluting balloon perform in the trial when compared with placement of a drug-eluting stent (DES) and balloon angioplasty for the treatment of ISR?
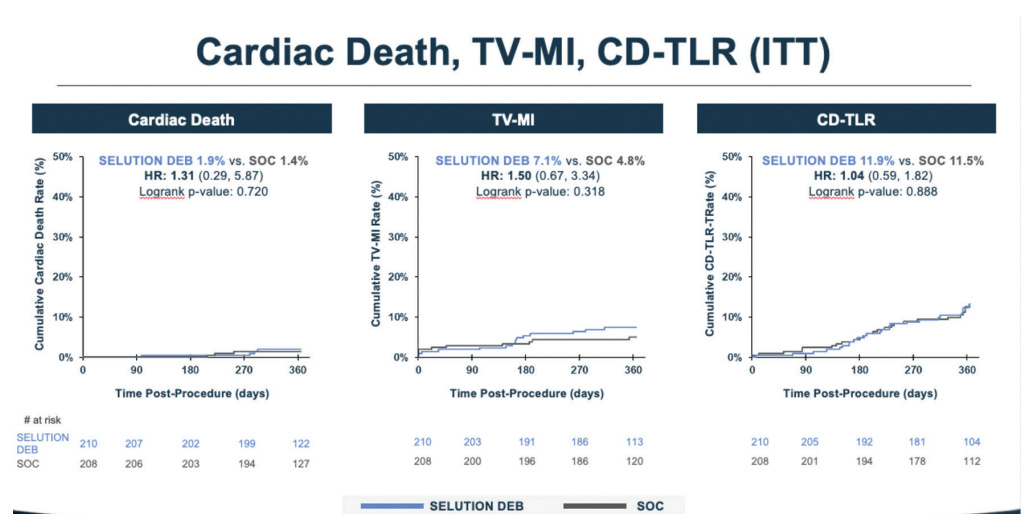
The key finding is that Selution was noninferior to the blended standard-of-care control group (80% DES and 20% balloon angioplasty). The observed difference in the primary endpoint, target lesion failure, was 1.7%, with rates of 14.5% for control versus 16.2% for Selution (Figure 1). The primary endpoint was a composite of cardiac death, target vessel myocardial infarction, or clinically driven target lesion revascularization (Figure 2).
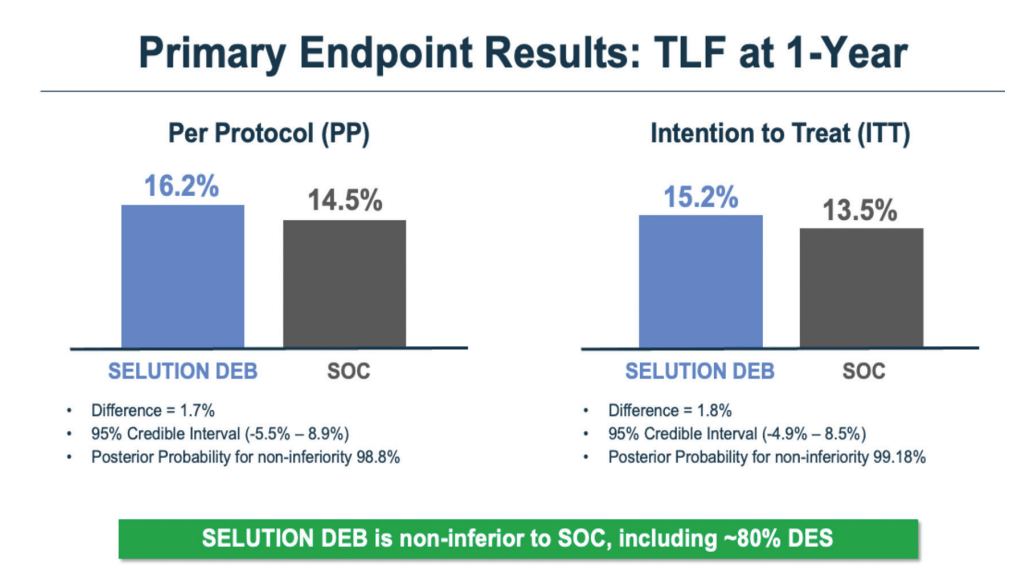
We used a Bayesian analysis, with a required posterior probability of at least 97.5% to demonstrate noninferiority, and we achieved approximately 98.8%, so the noninferiority result was quite robust.
An important aspect of this study is the inclusion of a DES control arm. This may be the only trial that includes DES in the control group, because once a device is approved, future trials will likely compare against that device rather than DES. The DES data are therefore very informative as we think about first-line treatment for ISR.
Even though the DES group was somewhat selected and were generally less complex cases, DES performed very well. Lesion preparation was rigorous and required intracoronary imaging, which arguably gave DES an advantage. Still, in single-layer ISR, there was about a 7% to 8% difference favoring DES over the drug-eluting balloon. Some operators may view that difference as acceptable if it means avoiding a second stent layer, while others may not. The important point is that we now have data to inform that decision. Longer-term follow-up (patients will be followed out to 5 years) may ultimately be even more informative than the one-year results.
Can you describe the Selution balloon and why the use of sirolimus is attractive?
Most prior drug-eluting balloon trials have used paclitaxel. We don’t know that paclitaxel is inferior, but there is a sense that the therapeutic window for sirolimus and other limus drugs may be wider. That belief is based partly on pharmacologic data and partly on extensive experience with drug-eluting stents, where limus-based drugs have generally shown favorable safety profiles. Also, while I’m not an expert in this area, paclitaxel’s crystalline structure may increase the potential for downstream particulate embolization. This has been demonstrated experimentally, although it hasn’t clearly translated into clinical safety issues. Still, it’s a theoretical concern and another reason why limus-based balloons are attractive to explore.
The challenge is that paclitaxel is easy to deliver. It is highly lipophilic and rapidly absorbed. Sirolimus is quite different. It is not lipophilic and has reversible binding, so it needs prolonged exposure to achieve effective tissue uptake.
The Selution balloon addresses this by packaging sirolimus into very small polymer-based microspheres, about four micrometers in size. These are loaded onto the balloon and protected by a phospholipid bilayer as the device is advanced through the vasculature. When the balloon is deployed, the phospholipid layer adheres to the endothelium, allowing prolonged exposure of the microspheres and sustained drug transfer. At least 30 seconds of balloon inflation is required, with 60 seconds recommended, and this protocol was used in the trial. Pharmacokinetic data demonstrate that the Selution achieves therapeutic tissue levels at 90 days comparable to those seen with drug-eluting stents, while delivering a similar drug dose.2
Keep in mind the drug-eluting balloon is simply a delivery vehicle. It is not intended to treat the lesion mechanically. Lesion preparation should be done beforehand, including any high-pressure inflations. The drug-eluting balloon should then be deployed at nominal pressure, just enough to appose the balloon to the vessel wall without causing additional dissections or disrupting the protective membrane.
What about deliverability of the Selution balloon in tortuous vessels or calcified segments? Any concerns there?
That’s exactly why the protective layer is so important. It protects the drug during delivery, even through calcified or tortuous anatomy, and seems to hold up well.
The design of the trial was carefully thought out. Can you walk us through that?
The challenge was how to design a trial that reflected what was actually happening in the U.S. At the time, and still today, most data showed that for in-stent restenosis, repeat implantation of a drug-eluting stent was the best therapy, and that was the guideline-recommended approach in both the U.S. and Europe.
However, in practice, many interventionalists are increasingly reluctant to keep adding layers of stent. There is concern that stenosis begets stenosis, leading to more procedures and more complications over time. So even though repeat DES is the approved and recommended therapy, it wasn’t always what was happening clinically.
We have data from the National Cardiovascular Data Registry showing that up to 2017, about 80% of ISR cases were treated with repeat DES, while about 20% were treated without a DES, most commonly with balloon angioplasty.3 We felt a blended control group that reflected that reality was the most appropriate and pragmatic trial design.
Ideally, we would have randomized against DES alone. But given the lack of equipoise among operators, enrollment would have been very challenging. And even if we were able to enroll patients, they likely would have been limited to relatively simple, single-layer ISR cases, exactly the group that would give us the least information about the potential value of a drug-eluting balloon.
Is there a practical limit as to how many stent layers should be placed in a coronary artery?
The short answer is that nobody really knows, but there is clear reluctance to continue adding layers. Even within the trial, we had to cap the balloon angioplasty control arm at 20%. If it exceeded that, we would no longer have been able to test noninferiority and instead would have had to demonstrate superiority. That cap was reached early, after only about 40% of patients were enrolled, so the remainder of the trial compared the Selution drug-eluting balloon primarily against DES.
Despite that, operators were still reluctant to place additional stent layers. There are observational data suggesting that as you move from a second to a third layer, the risk of recurrent events increases. These aren’t randomized data, but combined with concerns about theoretical loss of vasomotor function, side-branch jailing, and overall event risk, they contribute to the hesitation around additional stenting.
The control strategy was selected by the operator prior to randomization. Why is that important?
That is a critical design element. The operator had to decide up front, before randomization, what therapy they would use if the patient were assigned to the control arm. If that decision were made after randomization, it would introduce significant bias.
Another option would have been to stratify randomization based on operator choice, but because we had to closely monitor and cap the angioplasty arm, and we already had several stratification variables, we felt it was cleaner to have the operator choose in advance. That way, comparisons were fair: Selution versus balloon angioplasty among patients selected for balloon angioplasty, and Selution versus DES among patients selected for DES.
As experience grows, do you think outcomes could improve further with better lesion preparation?
That’s a very important question. Only about half of patients in the trial received cutting or scoring balloons during preparation. We suspect that may facilitate drug transfer into the vessel wall. We plan to analyze outcomes based on preparation strategy, and if results are better with cutting or scoring balloons, it could suggest that performance in real-world practice might actually exceed what we observed in the trial. Given the roughly 50/50 use of these devices, the analysis should be informative.
Can you comment on the role of intravascular imaging in this trial?
Imaging was required, and that is particularly important in ISR. You need to understand why restenosis occurred. Often it is due to stent under expansion. Until that is corrected, neither a drug-eluting balloon nor another stent will be effective. Achieving full stent expansion may require high-pressure inflations or calcium modification, and imaging helps guide that process. We also want to examine whether adequate expansion was consistently achieved before drug delivery.
Was geographic miss a concern?
We were careful about that in the trial protocol. The balloon had to extend beyond the prepared segment, and Selution balloon sizing made that feasible (coronary lengths range from 10 to 40 mm). While we only had angiographic follow-up in a small subset, baseline imaging may allow us to identify cases at risk for geographic miss, even if we can’t fully assess its downstream impact.
Any practical tips for using Selution?
Well, the most important point, which we’ve already mentioned, is lesion preparation. You want to achieve less than 30% residual stenosis and make sure the stent is fully expanded. Intravascular imaging is helpful here, particularly for sizing. The drug-eluting balloon must be well apposed to the vessel wall. Finally, balloon length matters. You want to be sure you have covered the entire segment that was treated during lesion preparation.
How do you see drug-eluting balloons fitting into practice going forward?
Assuming Selution receives FDA approval, there would then be two drug-eluting balloons available in the U.S. for in-stent restenosis. In cases of recurrent ISR, where there are already two layers of stent, I think drug-eluting balloons will clearly become the first choice. Plain balloon angioplasty, which is still used in roughly 20% of cases, will probably fall out of favor. As we and others have shown, similar to the Boston Scientific AGENT studies with paclitaxel balloons, balloon angioplasty performs very poorly for ISR and really should not be used as a treatment option.
Where things are less certain is the first event, single-layer ISR. I suspect practice will be split, with some operators still willing to place a second stent and others more inclined to use a drug-eluting balloon and accept the possibility of recurrence.
How might the use of Selution help move toward fewer implants overall?
While we did not see this in our study, because unfortunately there is already something left behind in ISR, there was another study presented at TCT 2025 by Christian Spaulding,4 who led the SELUTION DeNovo randomized trial (NCT04859985), and those results were also very impressive for the drug-eluting balloon. One of the key lessons from that work is just how important lesion preparation is, and getting comfortable with angioplasty results that may look acceptable rather than perfect but still perform well over time. That’s something we would need to relearn in the United States, where nearly everyone gets a stent.
It may actually be an even more exciting direction for drug-eluting balloons than ISR. If we can use a drug-eluting balloon in de novo disease, that would be very meaningful. We are currently enrolling a U.S. trial with Selution for de novo lesions in small vessels (NCT05946629) and expect to have those results in about a year.
*The Selution SLR currently has FDA Breakthrough Device Designation, conditional Investigational Device Exemptions (IDEs), and has also obtained CE Mark recognition.
References
- Cutlip DE. Sirolimus-eluting balloon versus repeat drug-eluting stenting or balloon angioplasty for in-stent restenosis. Presented as part of TCT 2025; October 26, 2025; San Francisco, CA. https://www.tctmd.com/slide/sirolimus-eluting-balloon-versus-repeat-drug-eluting-stenting-or-balloon-angioplasty-stent
- Tanaka T, Kawakami R, Shiraki T, et al. A pharmacokinetic comparison between three drug delivery devices in porcine coronary arteries. Cardiovasc Revasc Med. 2025 Sep 11; [Epub ahead of print]. doi:10.1016/j.carrev.2025.09.001
- Moussa ID, Mohananey D, Saucedo J, et al. Trends and outcomes of restenosis after coronary stent implantation in the United States. J Am Coll Cardiol. 2020 Sep 29; 76(13): 1521-1531. doi:10.1016/j.jacc.2020.08.002
- Spaulding CM. One-year results of the SELUTION DeNovo trial comparing a strategy of PCI with a sirolimus-eluting balloon and provisional stenting versus systematic DES implantation to treat denovo coronary lesions. Presented as part of TCT 2025; October 26, 2025; San Francisco, CA. https://www.tctmd.com/slide/one-year-results-selution-denovo-trial-comparing-strategy-pci-sirolimus-eluting-balloon-and
Find More:
Renal Denervation Topic Center
Cardiovascular Ambulatory Surgery Centers (ASCs) Topic Center
Grand Rounds With Morton Kern, MD
Peripheral Artery Disease Topic Center











