How Did Michigan Become the National Leader in Intracoronary Imaging-Guided PCI?
© 2025 HMP Global. All Rights Reserved.
Any views and opinions expressed are those of the author(s) and/or participants and do not necessarily reflect the views, policy, or position of Cath Lab Digest or HMP Global, their employees, and affiliates.
Sabina Kumar, DO1,4; Devraj Sukul, MD MSc2; Ryan Madder, MD3; Milan Seth2; Jay Mohan, DO1; Hitinder Gurm, MD2; Eric Cantey, MD2; Mark Zainea, MD1
1Division of Cardiovascular Medicine, Michigan State University: McLaren Macomb-Oakland, Mount Clemens, Michigan;
2Division of Cardiovascular Medicine, Department of Internal Medicine, University of Michigan, Ann Arbor, Michigan;
3William Beaumont University Hospital, Corewell Health East, Royal Oak, Michigan;
4Department of Cardiology, The Mount Sinai Hospital, New York, New York
The authors can be contacted via Sabina Kumar, MD, at dr.sabinakumar@gmail.com.
The History of BMC2
The Blue Cross Blue Shield of Michigan Cardiovascular Consortium (BMC2) was formed in 1996 by a collaborative group of cardiologists. BMC2 is dedicated to improving the quality of care and outcomes for cardiovascular patients undergoing interventional cardiology procedures, vascular surgery procedures, and transcatheter valve replacement procedures in the State of Michigan. BMC2 consists of all 48 catheterization laboratories performing over 20,000 percutaneous coronary interventions (PCI) annually.
Detailed data are collected for every PCI performed using the National Cardiovascular Data Registry (NCDR) CathPCI data registry framework with additional data elements included to inform specific quality improvement initiatives. Collaborative-wide quality improvement goals are identified as a group, and pay-for-performance incentives are provided to physician groups and hospitals for meeting specific benchmarks.
Prior and current quality improvement measures have included radiation usage, pre- and post-PCI hydration, contrast-induced nephropathy rates, post-PCI bleeding, and vascular complications requiring transfusion. BMC2 has been successful in implementing quality improvement metrics statewide, which include reducing the rate of vascular access complications and those complications that require transfusions to below 1.5%, ensuring pre-procedure aspirin administration greater than 99%, and reducing the rate of contrast-induced nephropathy to below 3%.
Support for BMC2 is provided by Blue Cross Blue Shield of Michigan (BCBSM) and Blue Care Network as part of the BCBSM Value Partnerships program. Although BCBSM and BMC2 work collaboratively, the opinions, beliefs, and viewpoints expressed by the authors do not necessarily reflect the opinions, beliefs, and viewpoints of BCBSM or any of its employees. Further, BCBSM does not have access to BMC2 data, and all patient episodes occurring at engaged hospitals are included in the data registries, regardless of payer.
The Evidence for Intracoronary Imaging
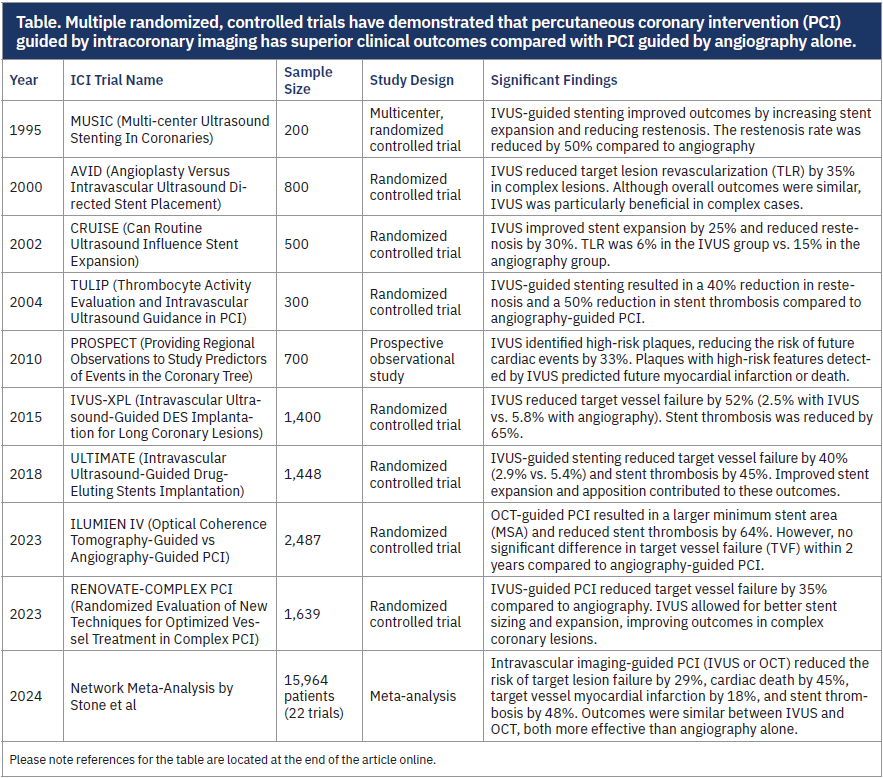
Multiple randomized, controlled trials have demonstrated that PCI guided by intracoronary imaging (ICI) has superior clinical outcomes compared with PCI guided by angiography alone (Table). The advantages of ICI-guided PCI include reductions in target vessel revascularization, stent thrombosis, myocardial infarction, and all-cause and cardiovascular mortality. The benefits of ICI-guided PCI have been demonstrated across multiple lesion subsets encompassing both complex and non-complex PCI. Despite the established benefit of ICI, in the United States, ICI was used in only 7-8% of PCIs from 2004-2018, compared with Japan, where it was used in 85% of cases during that same period.1 The recently published 2025 American College of Cardiology/American Heart Association guidelines for management of acute coronary syndromes give ICI-guided PCI a Class I recommendation for guiding PCI in more complex lesion subsets. The 2024 European Society of Cardiology’s guidelines for management of chronic coronary syndromes give ICI-guided PCI a Class I recommendation.
Why BMC2 Made ICI-Guided PCI a Quality Metric
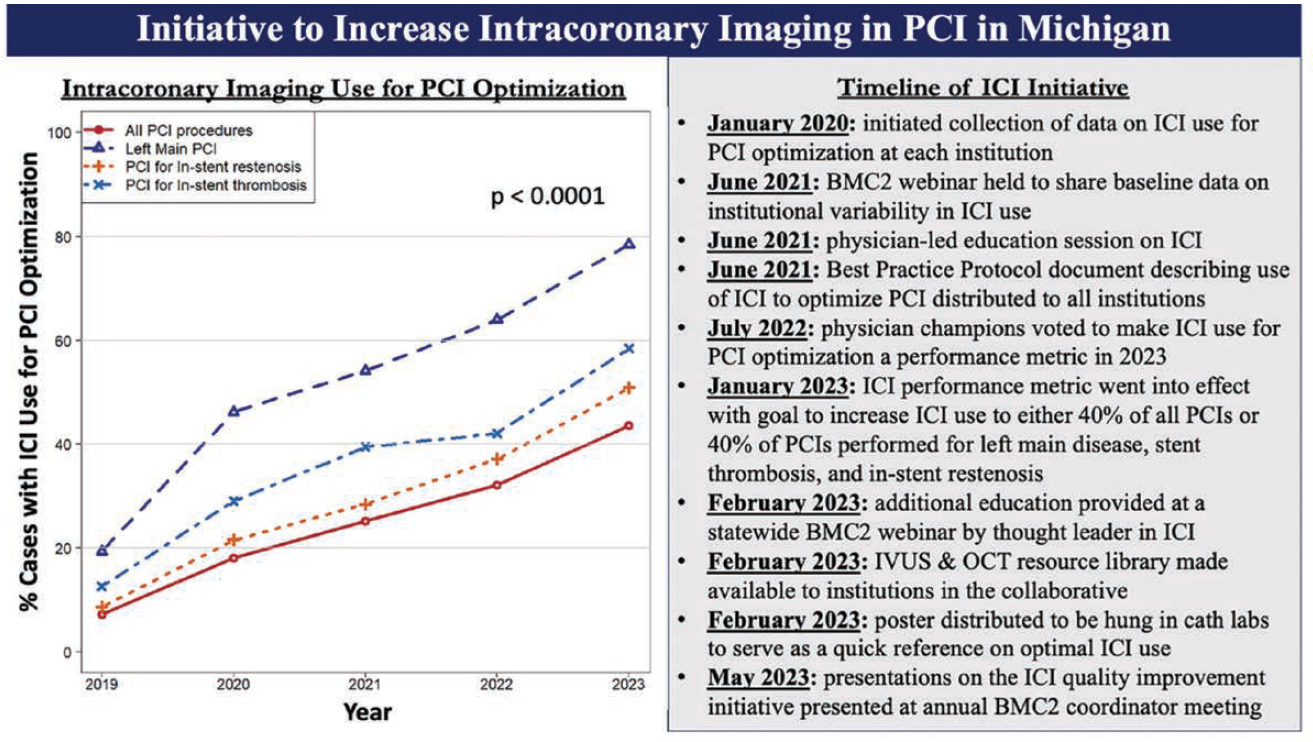
Based on the convincing results of multiple randomized, controlled trials and meta-analyses, BMC2 implemented a statewide quality improvement initiative in 2020 intended to increase the utilization of ICI to guide PCI. The steps taken in the initiative are shown in the Figure.1 In 2022, BMC2 physician champions voted to make ICI-guided PCI a performance metric for calendar year 2023. There was some debate as to whether specific measurements, including minimum stent area and/or distal reference lumen area, should be mandated in procedural reports. However, the committee ultimately decided to first encourage an increased use of ICI. The goal for the performance metric was the use of ICI to guide at least 40% of all PCIs or 40% of PCI performed for left main stenosis, in-stent restenosis, or stent thrombosis (Figure).
Reprinted with permission from Madder RD, et al. J Soc Cardiovasc Angiogr Interv. 2025 July; 4(7): 103710. doi:10.1016/j.jscai.2025.103710. Licensed under a Creative Commons Attribution 4.0 International license (https://creativecommons.org/licenses/by-nc/4.0/).
Comparing National Data With BMC2 Data
In 2019, the use of ICI-guided PCI in Michigan was approximately 7% across the 48 hospitals in the BMC2 consortium. However, following the implementation of the ICI quality improvement initiative by BMC2, ICI-guided PCI increased significantly, reaching 44% by 2023.2 This growth appears to outpace national trends, where ICI-guided PCI was only 6.6% in 2016, with a modest increase to 15.4% by 2020.3
Malik et al analyzed the National Readmission Database and found that among 1,328,517 PCI procedures, the median hospital use of ICI increased from 2.7% (IQR 0.6-7.7) in 2016 to 6.3% ( IQR 1.7-17.8) in 2020.3 Even more surprising was that in 2020, 86% of the hospitals had ICI capabilities but ICI imaging was used in only 6% of procedures.3
In Michigan, a subgroup analysis of the BMC2 data revealed even more remarkable improvements in ICI use among certain lesion subsets. For PCIs performed for left main disease, in-stent restenosis, and stent thrombosis, ICI-guided interventions increased from 19% to 79%, 9% to 51%, and 14% to 61%, respectively, between 2019 and 2023.
Using the BMC2 data, a Bayesian analysis identified several factors that were independent predictors of ICI use, including left main PCI, proximal left anterior descending (LAD) PCI, in-stent restenosis, surgical consultation prior to PCI, and mid- or distal LAD PCI. Conversely, factors associated with a lower likelihood of ICI optimization during PCI included prior coronary artery bypass graft surgery, New York Heart Association Class IV, left circumflex artery PCI, new-onset angina, ST-elevation myocardial infarction (STEMI), non-STEMI, the use of ventricular support, and increasing patient age.2
A Culture Change in Michigan Cardiac Cath Labs
There are various perspectives on why ICI has not been used routinely in cardiac catheterization labs in the United States. Some potential barriers include the additional time required to perform ICI during a PCI procedure, lack of adequate training, reimbursement issues, and expense.
At both community-based hospitals and academic institutions in Michigan, cath labs have widely adopted ICI-guided PCI as a more routine practice. This paradigm shift was achieved through a comprehensive initiative focused on the education of interventional cardiologists, cath lab staff, and fellows.
Staff training is important to ensure that the imaging equipment will be readily available for every case, streamlining operations and simplifying the process for operators. This change can be reinforced by demonstrating the value of ICI imaging to the team and setting the expectation that it will be used in every case.
Recognizing that cardiology fellows represent the future of interventional cardiology, it is crucial for attendings to adapt their practices with this rapidly evolving field. This included the integration of ICI imaging during PCI procedures and educating fellows on the importance of ICI-guided PCI. ICI should also be more fully incorporated into interventional cardiology conferences to support this learning.
Interventional cardiology is an ever-evolving field, and the future looks markedly different from the practices of the 1990s and early 2000s. The future of ICI will be increasingly shaped by artificial intelligence, which is expected to enhance both performance and interpretation, leading to broader adoption and more precise outcomes. It is essential for both experienced and younger physicians to continuously learn and adapt to new technologies to ensure the highest quality of patient care.
The authors can be contacted via Sabina Kumar, MD, at dr.sabinakumar@gmail.com.
References
1. Kuno T, Numasawa Y, Sawano M, et al. Real-world use of intravascular ultrasound in Japan: a report from contemporary multicenter PCI registry. Heart Vessels. 2019; 34: 1728-1739. doi:10.1007/s00380-019-01427-9
2. Madder RD, Seth M, Sukul D, Yelavarthy P, Pielsticker E, Gribar J, Kumar S, Zainea M, Croce K, Shlofmitz E, Wanamaker B, Gurm HS. Statewide initiative to increase intracoronary imaging optimization in PCI: a report from the BMC2 registry. J Soc Cardiovasc Angiogr Interv. 2025 July; 4(7): 103710. doi:10.1016/j.jscai.2025.103710
3. Malik AO, Saxon JT, Spertus JA, et al. Hospital-level variability in use of intracoronary imaging for percutaneous coronary intervention in the United States. J Soc Cardiovasc Angiogr Interv. 2023 May 19;2(4):100973. doi:10.1016/j.jscai.2023.100973
Table References
1. Serruys PW, de Jaegere P, Kiemeneij F, et al. A comparison of balloon-expandable-stent implantation with balloon angioplasty in patients with coronary artery disease. N Engl J Med. 1994; 331(8): 489-495. doi:10.1056/NEJM199408253310801
2. Russo RJ, Silva PD, Teirstein PS, et al. A randomized controlled trial of angiography versus intravascular ultrasound-directed bare-metal coronary stent placement (The AVID Trial). Circ Cardiovasc Interv. 2009; 2(2): 113-123. doi:10.1161/CIRCINTERVENTIONS.108.778647
3. Fitzgerald PJ, Oshima A, Hayase M, et al. Final results of the Can Routine Ultrasound Influence Stent Expansion (CRUISE) study. Circulation. 2000; 102(5): 523-530. doi:10.1161/01.CIR.102.5.523
4. Fujii K, Masutani M, Okumura M, et al. Intravascular ultrasound-guided versus angiography-guided stent implantation in complex coronary lesions: the TULIP Study. J Am Coll Cardiol. 2003; 41(2): 230-236. doi:10.1016/S0735-1097(02)02626-1
5. Stone GW, Maehara A, Lansky AJ, et al. A prospective natural-history study of coronary atherosclerosis. N Engl J Med. 2011; 364(3): 226-235. doi:10.1056/NEJMoa1002358
6. Hong SJ, Kim BK, Shin DH, et al. Effect of intravascular ultrasound-guided vs angiography-guided everolimus-eluting stent implantation: the IVUS-XPL randomized clinical trial. JAMA. 2015; 314(20): 2155-2163. doi:10.1001/jama.2015.15454
7. Zhang J, Gao X, Kan J, et al. Intravascular ultrasound–guided versus angiography-guided implantation of drug-eluting stents in all-comers: the ULTIMATE trial. J Am Coll Cardiol. 2018; 72(24): 3126-3137. doi:10.1016/j.jacc.2018.09.051
8. Ali ZA, Landmesser U, Maehara A, et al; ILUMIEN IV Investigators. Optical coherence tomography-guided versus angiography-guided PCI. N Engl J Med. 2023; 389(25): 2320-2332. doi:10.1056/NEJMoa2305861
9. Lee JM, Choi KH, Song YB, et al; RENOVATE-COMPLEX-PCI Investigators. Intravascular imaging–guided or angiography-guided complex PCI. N Engl J Med. 2023; 388(18): 1668-1679. doi:10.1056/NEJMoa2216607
10. Stone GW, Christiansen EH, Ali ZA, et al. Intravascular imaging-guided coronary drug-eluting stent implantation: an updated network meta-analysis. Lancet. 2024; 403(10429): 824-837. doi:10.1016/S0140-6736(23)02454-6










