Paradise™ Ultrasound Renal Denervation in Contemporary Practice
CLD talks with Behnam N. Tehrani, MD.
CLD talks with Behnam N. Tehrani, MD.
© 2025 HMP Global. All Rights Reserved.
Any views and opinions expressed are those of the author(s) and/or participants and do not necessarily reflect the views, policy, or position of Cath Lab Digest or HMP Global, their employees, and affiliates.
Can you tell us about your experience with the Paradise™ Ultrasound Renal Denervation System (uRDN)?
My clinical practice is primarily providing care for patients with complex coronary and peripheral vascular disease. The vast majority of these patients also have difficult-to-control hypertension. During my career, I followed the clinical trials pertaining to renal denervation closely, with particular attention to the RADIANCE program evaluating the Paradise™ ultrasound system (the RADIANCE-HTN SOLO, TRIO, and RADIANCE II trials) from Recor Medical. The 2023 pooled meta-analysis from these trials1 demonstrated clear signals related to efficacy and with excellent safety profiles. After the FDA approval of Paradise™ uRDN in November 2023, we were among the early adopters and we brought the Paradise™ uRDN system to the Inova Health System in April 2024. Since then, we have performed 28 uRDN cases, and we have found it to be a quite effective targeted therapy.
What clinical outcomes and blood pressure trends are you seeing in your patients?
The outcomes have been important and clinically meaningful. Among the patients where we have 90-day follow up data, we have observed a median blood pressure reduction of over 30 mmHg systolic and 10 mmHg diastolic, with a median reduction of 1.3 antihypertensive medications (n=24). Patients regularly tell us they objectively feel better, experiencing fewer symptoms such as shortness of breath and chest pain, resulting in a better quality of life. Equally as important, we have not encountered any procedure-related complications, such as major bleeding and vascular complications, renal hematomas, renal artery dissections, or perforations. Moreover, none of our patients have experienced a meaningful decline in renal function or required dialysis during follow-up. From the outset, we implemented a structured follow-up algorithm at 1, 3, 6, and 12 months, thereby ensuring that every patient undergoes standardized assessment of blood pressure measurements, medication burden, renal function, and renal artery anatomy to rule out iatrogenic stenoses.
In our early experience, we have observed a few common and noteworthy themes. First, patients typically mention within two weeks of the procedure that they no longer experience the wide swings in blood pressure that they did in the past. By about 90 days, most patients’ blood pressure readings plateau lower and stay steady. At that time, in collaboration with their primary team, we will begin to assess for potential de-escalation of their medication burden. The stability and consistency that patients experience in their blood pressures are often as meaningful to them as the absolute reduction. That being said, there is a certain degree of variability in blood pressure response between patients. Further research is needed to better understand predictors of sustained response to RDN. This will help us to further refine the technology so that we can deliver precision-based and patient-centered treatment to this population.
What is your overall approach to the uRDN procedure to encourage the best possible outcome?
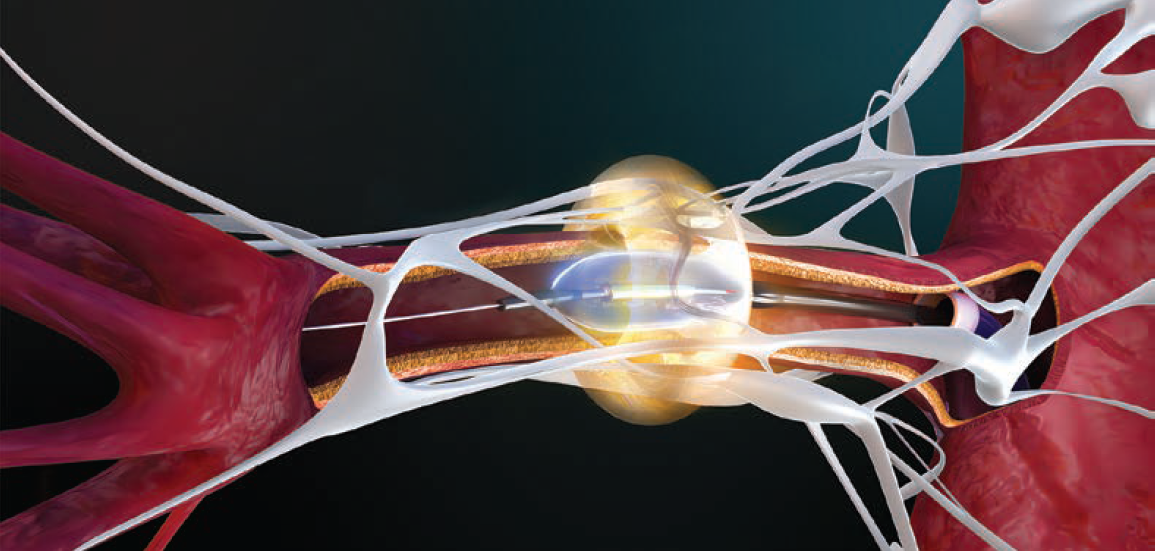
Pre procedure, we will review either a renal duplex or computed tomography angiography (CTA) with our radiologists to understand the presence or absence of accessory vessels as well as branch take-offs and tortuosity.2 A thorough imaging review helps to inform strategic planning and thereby streamlines the case, minimizes contrast utilization, and helps ensure that we are able to offer our patients complete denervation. We treat the main renal arteries and all accessory branches that fall within the 3-8 mm diameter range. It is also important to determine accurate vessel diameters so that we choose the appropriate balloon size to ensure that the ultrasound energy efficiently ablates the peri-arterial nerves. That attention to sizing and wall contact, as well as an appreciation that nerve distribution differs from proximal to distal, has been central to getting consistent responses.*
Can you address ease of use for this device?
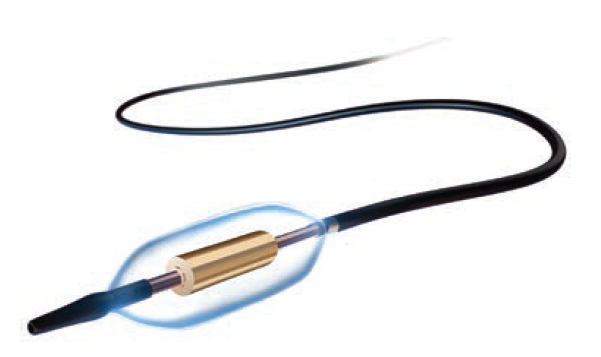
As an interventional cardiologist, I have found use of the Paradise™ uRDN to be intuitive. It is a balloon-on-a-wire workflow, which is our native language, and ultrasound-based RDN requires only 7-second treatments, about 3 times in each renal artery. The combination of a balloon-based platform and the consideration of balloon sizing and apposition is something that experienced interventional cardiologists will feel very comfortable with.
What are your thoughts on the safety profile of Paradise™ uRDN in your experience?
Our experience has shown this technology to be safe, without periprocedural complications and no rehospitalizations attributable to the therapy. Patients proceed through a standardized follow-up, including a 6-month duplex to screen for iatrogenic stenosis. To date, we have not sent anyone to dialysis or observed a clinically meaningful decline in renal function. In an integrated health system with the appropriate expertise, procedural discipline, and post-procedure follow-up structure, Paradise™ uRDN has shown a favorable risk profile.
Can you share some technical tips?
• It is very important to have a collaborative relationship with your radiology colleagues. We all review the renal duplex and CTA imaging together. A precise awareness of anatomy that includes accessory vessels, tortuosity, and branch take-offs can allow you to carry out an efficient case with minimal contrast and without any periprocedural complications.
• It is important to ascertain the true vessel diameter and to ensure full apposition, which is how the ultrasound energy is reliably delivered to the target nerves.
• Standardize your tools. A short 55 cm 7 French (Fr) internal mammary artery (IMA) or 7 Fr renal double curve (RDC) guide works for most renal anatomies; keep a coronary guide extension handy for difficult engagement. A non-hydrophilic wire such as a Balance Middle Weight (BMW) provides a soft, steerable tip with stable support to deliver the balloon.
• Agree on peri-procedural and pharmacotherapy before the patient is on the table. Using the right agents up front and best practices for vascular access and pain management will pay dividends.
• Close the loop with referring colleagues. After each case, it is very important to connect with the internist, primary cardiologist, nephrologist, endocrinologist, and other health care providers involved in the patient’s care. It is also advised to provide referring physicians a succinct but thorough description of the vessels treated and the post-procedural plan of care. This transparency builds trust and encourages constructive and professional collaborations.
What has your learning curve been like?
Appropriate and manageable. Early on, we developed and implemented a standardized pathway around pre-procedural imaging, equipment, and peri-denervation pharmacology in order to minimize variations in practice patterns. We also identified two super users from our lab who underwent training offered by Recor Medical. These team members staffed the first several cases with me and then disseminated the workflow to the broader team. That model has helped us scale without losing quality. We reinforce it with quarterly team education so new staff can get up to speed and experienced staff can refresh.
Tell us more about how these patients are coming to you. What does your selection and referral process look like?
Early on, referrals came mainly from general cardiology inside our system, but more public awareness has broadened the funnel. Today we receive direct referrals from nephrology, endocrinology, and internal medicine, as well as advanced practice providers who spot difficult-to-control hypertension during hospitalizations for other issues. We built advisories in our Epic EMR to prompt clinicians when a patient meets criteria for resistant hypertension and suggest a referral to our clinic. From there, patients undergo a structured evaluation with preventive and interventional cardiology.
What is involved in the pre-procedure evaluation?
We start by confirming true resistant hypertension and excluding common secondary causes. That work-up, which we try to accomplish within 45 days, includes screening for sleep apnea, renal artery stenosis (>30%), pheochromocytoma, thyroid disease, adrenal tumors, and aortic coarctation. Once secondary causes are ruled out and criteria are met, we begin the preparation process for RDN. We participate in shared decision-making with patients and their loved ones so expectations and logistics are clear.
How do you talk to patients about what they can expect from this procedure?
We emphasize to patients that RDN is not meant to be a substitute for optimal medical therapy. Rather it is an adjunctive strategy which will hopefully provide a positive and meaningful change to the trajectory of their cardiovascular health. The first change patients notice is stability in their readings, typically within two weeks. By three months, blood pressure reads will typically plateau and steady out. That being said, there may be variability in response between patients. I also share information with patients about the safety track record in our program.
What do you anticipate for the future of your practice around these patients and this procedure?
I see three near-term shifts. First, as national coverage solidifies, the approval pathway should streamline. Commercial payers will likely follow, thus broadening access for patients who have struggled to get coverage. Second, I anticipate a national push for multidisciplinary hypertension programs where an interventional option like the Paradise™ uRDN is embedded within a broader care model, rather than siloed. This way, patients will be able to proceed through a coordinated pathway from evaluation to follow-up. Finally, large post-market registries will increase our understanding of responders to therapy, and potentially predictors of favorable responses. These registries will allow for insights into populations under-represented in the trials (e.g., more advanced kidney disease, complex presentations such as heart failure, and diverse demographics). The knowledge garnered from large and enriched studies will inform our understanding of how to best apply this therapy to more complex patient populations.
Any final thoughts?
Ultrasound renal denervation, in our program, has been a strong addition to our care armamentarium. In our experience, the technology has been safe, reliable, and well tolerated, with no procedure-related complications and no major adverse events requiring rehospitalization. We were one of the very first centers to bring on Paradise™ uRDN. The Recor Medical team is professional and they have been great to collaborate with, providing terrific overall support. This technology has been a game-changer for our health system and for our patients.
References
1. Kirtane AJ, Sharp ASP, Mahfoud F, et al; RADIANCE Investigators and Collaborators. Patient-level pooled analysis of ultrasound renal denervation in the sham-controlled RADIANCE II, RADIANCE-HTN SOLO, and RADIANCE-HTN TRIO trials. JAMA Cardiol. 2023 May 1; 8(5): 464-473. doi:10.1001/jamacardio.2023.0338
2. Treatment site may vary with anatomy. Please refer to Paradise Catheter IFU for treatment strategy after accessory vessels.
This article is sponsored by Recor Medical.
Dr. Tehrani is a paid consultant of Recor Medical, Inc., and has been compensated for his time and expertise in participating in this content. The views expressed in the article are his own and based on his own personal experience with the Paradise System. Results may vary.
© 2025 Recor Medical, Inc. All rights reserved. RECOR MEDICAL, PARADISE, HYDROCOOLING, SONOWAVE 360, RADIANCE, the GPS logo, and the Swirl logo are registered trademarks in the EU and other countries, RECOR and the RADIANCE logo are also trademarks of Recor Medical, Inc. The Paradise System is FDA approved and indicated to reduce blood pressure as an adjunctive treatment in hypertension patients in whom lifestyle modifications and antihypertensive medications do not adequately control blood pressure. See full ISI at the end of the deck.
Paradise is a registered trademark of Recor Medical, Inc.
The Paradise System is FDA approved in the United States, is CE marked and approved for sale in markets where the CE mark is accepted per approved indications for use, and received manufacturing and marketing approval in Japan.











