When All Else Fails: Devices to Optimize PCI in Calcified and Tortuous Vessels
© 2025 HMP Global. All Rights Reserved.
Any views and opinions expressed are those of the author(s) and/or participants and do not necessarily reflect the views, policy, or position of Cath Lab Digest or HMP Global, their employees, and affiliates.
Boskey Patel, DO, FACC, FSCAI
Hartford HealthCare; Heart & Vascular Institute, Hartford Hospital, Hartford, Connecticut
Calcified coronary lesions remain a formidable opponent in the cath lab, despite the many advancements made in the field to overcome the challenges they pose. This is especially true in tortuous vessels, and often these lesions pose a greater challenge during a transradial approach. Fortunately, operators have an ever-evolving arsenal of interventional tools to help us obtain and maintain adequate support during complex percutaneous coronary intervention (PCI).
The Runthrough® NS Izanai™ Coronary Guidewire and the Takeru™ PTCA Balloon Dilatation Catheter from Terumo Interventional Systems are two such devices, designed to facilitate lesion access, preparation, and optimization. The Runthrough® NS Extra Floppy Coronary Guidewire is the de facto workhorse wire for the majority of interventionalists across the United States. The Izanai™ guidewire was developed using the same joint-duo core technology as the Extra Floppy, but the Izanai™ has a nitinol tip and enhanced “M-coat” hydrophilic coating, which allows it to be more trackable and pushable in tortuous arteries. In fact, the Izanai™ guidewire’s high lubricity means less force is required to push the wire through the vessel (2.45 grams) versus the Extra Floppy (3.87 grams). The white shaft color option of the Izanai™ also makes this wire a front-line contender for bifurcation PCIs or any case where more than one wire is required.
The Takeru™ PTCA Balloon Dilatation catheter similarly allows for best-in-class pushability and steerability, a crucial advantage in complex and calcific coronary lesions. Its low-entry crossing profile is key to its ability to navigate tortuous, calcific lesions. The Takeru™’s semicompliant option is well-known for its ability to easily open up stent struts in a rewired, jailed branch after stenting across the main vessel in a bifurcation PCI. What is less appreciated, however, is the versatility of the noncompliant (NC) Takeru™ balloon in both pre- and post-dilation, specifically in calcified stenoses. The Takeru™’s trackability also means that it can more easily navigate tortuous vessels to reach distal stents for post-dilation, as illustrated in the case below. Finally, as interventional cardiology moves toward more environmentally friendly, sustainable, and cost-effective cath lab practices, it is worth highlighting the tight re-wrap of the Takeru™ balloons, which allows interventionalists to reuse the balloon for either the same vessel or for another vessel during the same procedure. Our case highlights these benefits nicely.
Clinical Case
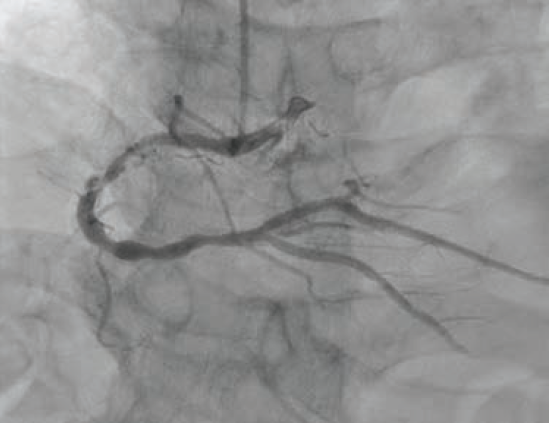
An 80-year-old male with hypertension, dyslipidemia, type 2 diabetes mellitus, chronic kidney disease stage IIIa, and known coronary artery disease (CAD) with recent PCI to the left anterior descending (LAD) artery presented to our emergency department (ED) with recurrent unstable angina. Ten days prior, he underwent coronary angiography for unstable angina and was found to have a 90% mid-LAD lesion that was stented with a 2.5 mm x 28 mm Synergy™ drug-eluting stent (DES) (Boston Scientific). Also present was severe calcific disease of the proximal and mid right coronary artery (RCA) (Figure 1); the plan was for staged PCI of the RCA in 4 weeks. However, prior to the planned procedure and due to recurrent unstable angina, the patient was sent back to the hospital by his primary care physician. On arrival, his electrocardiogram showed no ischemic changes, and serial high-sensitivity troponins were minimally elevated and flat.
The patient was brought to the lab for intravascular ultrasound (IVUS)-guided PCI of the RCA via a right radial approach, with calcium modification via intravascular lithotripsy (IVL) or atherectomy as needed.
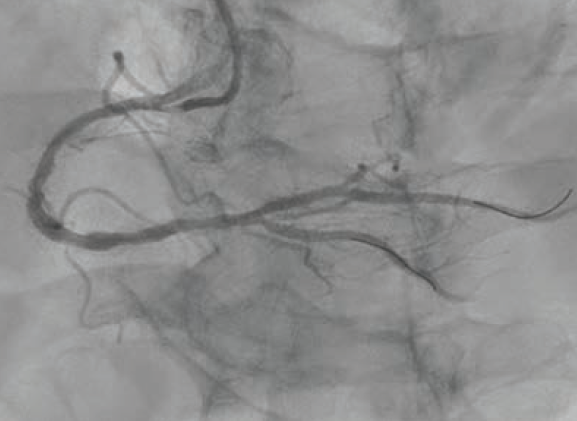
A 6 French AL 0.75 guide catheter was used to engage the ostium of the RCA. A Runthrough® NS Extra Floppy coronary guidewire was placed in the right posterolateral branch, while a Runthrough NS Izanai™ wire coronary guidewire was advanced to the right posterior descending artery (RPDA) as a buddy wire. Initial predilation was attempted with a 2.5 mm x 15 mm NC Euphora™ balloon (Medtronic), but this would not pass the lesion. A 6 French LiquID® guide catheter extension (Seigla Medical) was introduced for additional guide support. Despite this, the 2.5 mm NC Euphora™ was still unable to reach the area of interest without pushing out the guide. A 2.5 mm x 15 mm NC Takeru™ PTCA balloon dilation catheter was used instead, and was successfully brought to the diseased segment and inflated to high pressure. The rest of the proximal and mid vessel was also pre-dilated with the same balloon. We still could not bring the 6 French Opticross™ HD IVUS catheter (Boston Scientific) to the desired area due to the calcific nodule. Therefore, a 2.5 mm x 12 mm C2+ IVL balloon (Shockwave Medical) was used to deliver 70 total pulses to the mid RCA. This was followed by a 3.0 mm x 12 mm C2+ IVL balloon, with which 90 pulses were administered at 4-6 atmospheres (atm) each. Angiography showed reasonable luminal gain (Figure 2).
Further predilation was performed with a 3.0 mm x 21 mm NC Takeru™ PTCA balloon dilation catheter, after which the 6 French IVUS catheter was finally able to traverse the diseased segment.
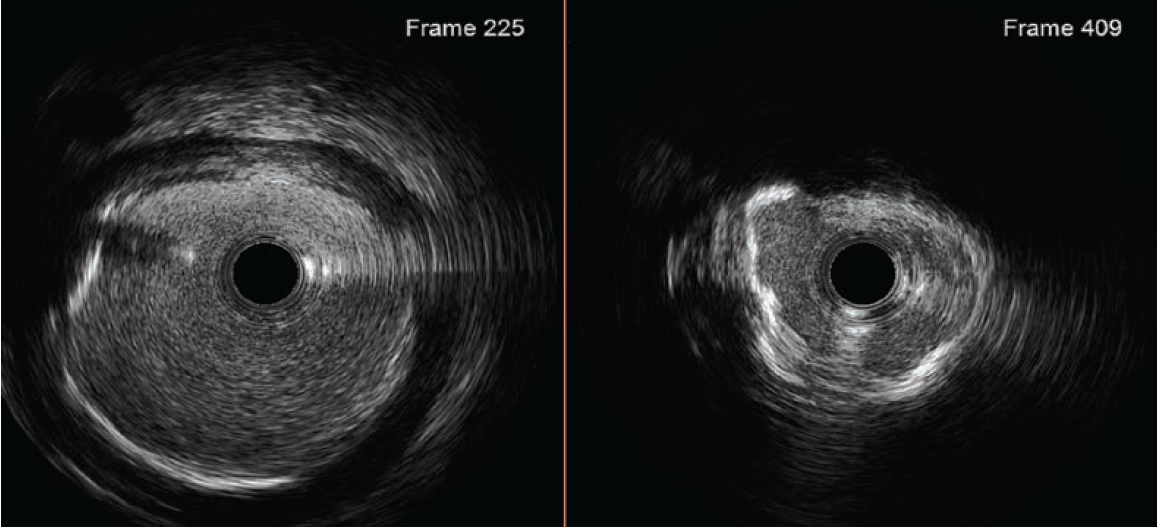
Based on the distal reference diameter of 3.6 mm (Figure 3), we tried to perform one more round of pre-dilation with a 3.5 mm Emerge™ Monorail PTCA dilatation catheter (Boston Scientific). This did not cross, and resulted in kinking of the LiquID® guide catheter extension. Both were removed, and a 6 French GuideLiner™ Coast™ Catheter (Teleflex) was inchwormed into the mid vessel utilizing the used 3.0
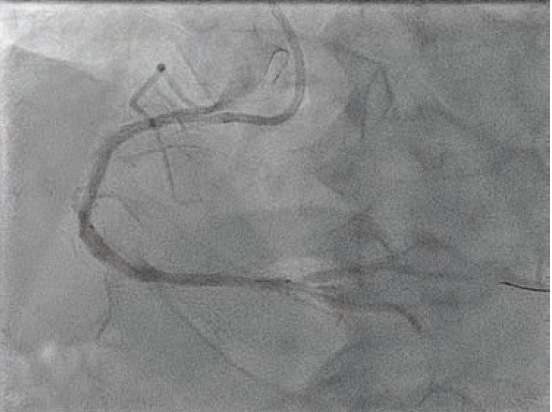
mm NC Takeru™ PTCA balloon dilation catheter as an anchor. More aggressive pre-dilation was performed with the 3.0 mm NC Takeru™ in the mid segment. Then, a 3.5 mm x 48 mm Synergy™ XD DES was successfully positioned in the mid-distal vessel (Figure 4) and deployed at nominal pressure.
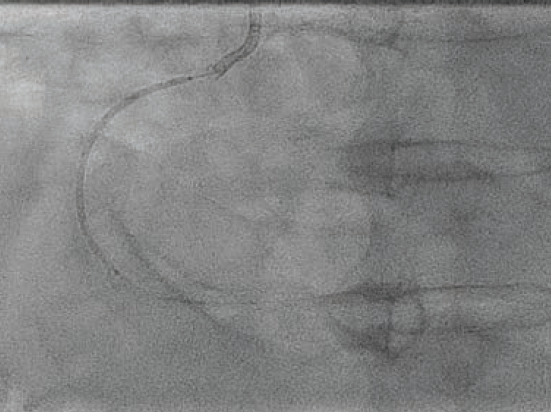
We attempted to post-dilate the stent with a 3.5 mm x 15 mm NC Euphora™ and then a 3.5mm x 15 mm NC Trek Neo™ RX coronary dilatation catheter (Abbott), but neither was able to navigate the mid vessel curve and reach the distal stent. Therefore, a 3.5 x 15 mm NC Takeru™ was used instead, and this was passed with ease into the distal stent, where post-dilation was performed to high pressures. A second, 4.0 mm x 48 mm Synergy™ DES was positioned across the proximal to mid vessel, with 2-4 mm distal overlap (Figure 5), and deployed at 12 atm. We attempted to post-dilate with a 4.0 mm x 15 mm NC Euphora™, but despite aggressive balloon preparation, it was unable to advance beyond the bend in the mid vessel to the overlapped segment. A 4.0 mm x 21 mm NC Takeru™ PTCA balloon was therefore used to perform post-dilation of the overlapped segment and the rest of the 4.0 mm stent.
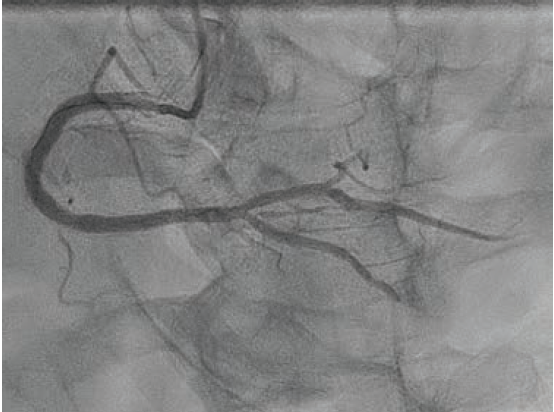
Final angiography demonstrated excellent angiographic results, with full expansion of stents and no evidence of dissection (Figure 6). There was TIMI-2 flow in the distal RPDA that improved after administration of intracoronary nitroprusside via a Sasuke® dual-lumen microcatheter (Asahi Intecc). Total contrast volume was 81 mL. The patient was discharged the next day with no complications. At one-month follow-up, the patient was still doing well, with no further symptoms of angina.
This case is sponsored by Terumo Interventional Systems.
PM-09873
Find More:
Renal Denervation Topic Center
Cardiovascular Ambulatory Surgery Centers (ASCs) Topic Center
Grand Rounds With Morton Kern, MD
Peripheral Artery Disease Topic Center
Go to Cath Lab Digest's Current Issue
Go to the Journal of Invasive Cardiology Issue










