Preventing Venous Thromboembolism After Foot and Ankle Surgery: A Rapid Review
Venous thromboembolism (VTE), including deep vein thrombosis (DVT) and pulmonary embolism (PE), are rare but potentially devastating complications after foot and ankle surgery.1 Mitigating these events can be challenging for foot and ankle surgeons given the relatively limited amount of high-quality data to guide VTE prevention in this population. The American Orthopaedic Foot & Ankle Society (AOFAS) highlights insufficient data to recommend for or against routine VTE prophylaxis in their 2020 position statement, stating future research is both “necessary and encouraged.”1 Fleisher and colleagues published the previous clinical consensus statement of the American College of Foot and Ankle Surgeons (ACFAS), which guides surgeons to address modifiable risk factors including using mechanical prophylaxis, early weight-bearing and consideration of chemical prophylaxis.2 One’s decision to prophylactically anticoagulate patients following foot and ankle surgery therefore begins with individualized risk/benefit stratification. This focused review discusses literature updates, summarizes current guidelines, and provides a suggested framework for VTE mitigation in the foot and ankle surgery patient.
Key Pathophysiology and Epidemiology
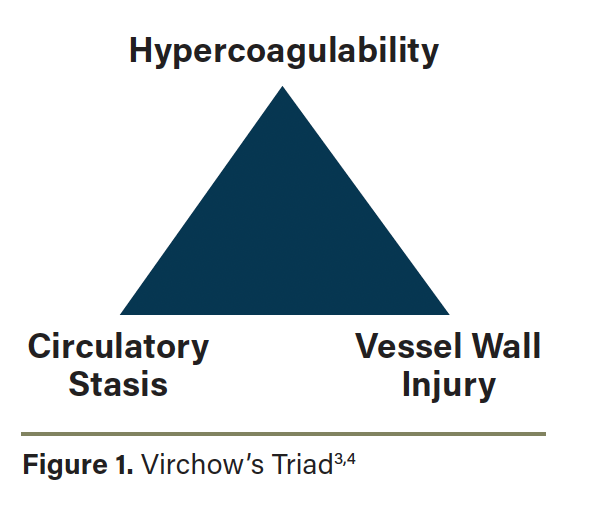
Rudolf Virchow first described the pathophysiologic mechanism of VTE in the mid-1800s. This concept has since been termed Virchow’s Triad (Figure 1).3 The triad includes 3 foundational mechanisms including damage to a vessel wall, circulatory stasis, and hypercoagulability.4 While the absolute risk may be small, foot and ankle surgery patients do have an increased risk for VTE secondary to the surgical insult itself, tourniquet use, and prolonged immobilization, in addition to patient-specific factors that may coexist.4
According to the literature, VTE incidence following foot and ankle surgery is considered low. Jameson and team retrospectively reviewed nearly 90,000 patients and reported a less than 0.3% rate of symptomatic VTE after foot and ankle surgery.5 Previously, Solis and colleagues reported a noticeably higher rate of postoperative DVT at 3.5%, though these investigators performed routine venous duplex ultrasound (US) exams on all patients.6 This study captures the true rate of DVT incidence, though it should be noted that many cases were asymptomatic, which may not require treatment per current guidelines.7 Rate of DVT may be related to the anatomic location of the procedure. A study by Heijboer and coworkers compared the rate of VTE and adverse bleeding events among 2 matched cohorts of 5,286 patients undergoing below-knee procedures with and without chemoprophylaxis.8 They identified an increase in the rate of VTE as one moved more proximally within the foot and ankle, including the forefoot (0.8%), hindfoot/ankle (1.4%), and lower leg (3.4%), among patients without chemoprophylaxis. The study also found an analogous increase among patients receiving chemoprophylaxis who underwent procedures to the forefoot (0.2%), hindfoot/ankle (0.4%), and lower leg (1.0%), and demonstrated a 3-fold reduction in the rate of VTE when using chemoprophylaxis but a 2-fold increase in bleeding events.8
Shibuya and colleagues reported an incidence of DVT and PE of 0.28% and 0.21%, respectively, following foot and ankle trauma.9 Distal leg fracture has been associated with ≤1% rates of symptomatic VTE across operative and nonoperative populations in the absence of chemoprophylaxis.10,11 Lower leg casting confers greater thrombogenicity, however, with symptomatic VTE occurring in 2% of casted patients with various injuries in one meta-analysis.12 Compared to the major orthopedic literature, VTE rates in foot and ankle surgery are lower (0.42% total VTE, including 0.27% DVT and 0.15% PE)13 and not as clearly augmented by VTE prophylaxis.14 Low and high estimated rates of symptomatic VTE after total ankle arthroplasty were 0.46% and 9.8% in one review.15
Achilles tendon rupture may confer the greatest VTE risk among foot and ankle populations, independently of operative versus nonoperative management.16 A secondary analysis of a randomized controlled trial (RCT) in patients with nonoperatively managed Achilles tendon rupture found a 47% rate of ultrasound-detected DVT by 8 weeks post-rupture in the absence of chemoprophylaxis.17 Additionally, DVT rates did not significantly reduce with early controlled motion versus immobilization. A meta-analysis investigating VTE risk after foot and ankle surgery found a clinical VTE rate of 7% and a radiologic VTE rate of 35% with Achilles tendon rupture.14 A Danish registry analysis of all Achilles tendon rupture patients across a nearly 20-year period identified 1.36% who required hospitalization for VTE within 180 days, and VTE rates were higher among older nonoperatively managed patients.18
General and Nonpharmacologic VTE Prophylaxis Modalities
Recommendations support a multimodal approach to VTE prevention after foot and ankle surgery, including routine preoperative assessment for VTE risk and optimizing modifiable risk factors including perioperative hydration status, perioperative mechanical prophylaxis, and early mobilization.2,19
Based on the literature, if possible, allowing early weight-bearing with >50% body weight on the operative extremity during ambulation decreases VTE risk.20 Postoperatively, early ambulation may minimize VTE risk, though this may be difficult following foot and ankle surgical procedures.19 Encouraging patients to complete non-weight-bearing passive exercise and range of motion may be beneficial. Advising immobilized patients to wiggle their toes and plantarflex and dorsiflex their ankles in a resting position can improve circulation.21
Perioperative mechanical VTE prophylaxis is paramount. Per the European guidelines on perioperative venous thromboembolism prophylaxis, all patients undergoing surgery should have intermittent pneumatic compression (IPC) devices placed on lower extremities across preoperative, intraoperative and postoperative phases of care to reduce VTE risk.19 Some IPC devices can be sent home with patients at discharge as a form of VTE prophylaxis. It is especially recommended that patients at high risk of bleeding postoperatively use IPC devices, when not optimal candidates for chemoprophylaxis.19 Unfortunately, current data suggests the devices must be worn consistently for approximately 18 hours per day in order to have a substantial prophylactic effect.22 The efficacy of this form of VTE prophylaxis is therefore often limited due to patient adherence to recommendations. Additional limitations to successful mechanical VTE prophylaxis include device fit for patients with larger body habitus or severe lymphedema. According to the National Institute for Health and Care Excellence (NICE), IPC are contraindicated in patients with severe forms of peripheral vascular disease (PVD), peripheral neuropathy, leg edema, or local skin conditions to the lower extremities.23 Mechanical prophylaxis is a useful adjunct in decreasing VTE risk following foot and ankle surgery, but is not an appropriate prophylaxis monotherapy in higher risk patients.2
Pharmacologic VTE Prophylaxis: A Patient-Centered Approach in Foot and Ankle Surgery
The decision to offer pharmacologic VTE prophylaxis in addition to general and nonpharmacologic modalities is patient-specific in foot and ankle surgery, and high-quality data are needed before strong recommendations can be made per available guidelines.2,19,24 In one robust propensity-matched retrospective analysis including >10,000 patients who underwent surgery distal to the knee, anticoagulant prophylaxis resulted in a 3-fold reduction in symptomatic VTE (0.7% vs. 1.9%) but a 2-fold increase in bleeding events (2.2% vs. 1.0%).8 A 2017 Cochrane meta-analysis of clinical trials in adult patients with lower-limb immobilization following injury determined symptomatic VTE was significantly lower with low-molecular weight heparin (LMWH) prophylaxis than with placebo or no prophylaxis (OR 0.40, 95% CI 0.21–0.76, n=2924), though the authors did not identify a significant difference in PE incidence.25 A 2019 meta-analysis identified only 6 prospective studies assessing VTE chemoprophylaxis in foot and ankle surgery for inclusion (N=1600). While VTE rates significantly reduced with chemoprophylaxis, event rates were low and symptomatic events were rare: only one nonfatal PE, no fatal PE, and no change in all-cause mortality were observed.26 These data highlight the relatively fine risk-benefit balance of pharmacologic VTE prophylaxis in foot and ankle surgery and the need for patient-specific decision-making.
VTE Risk Stratification
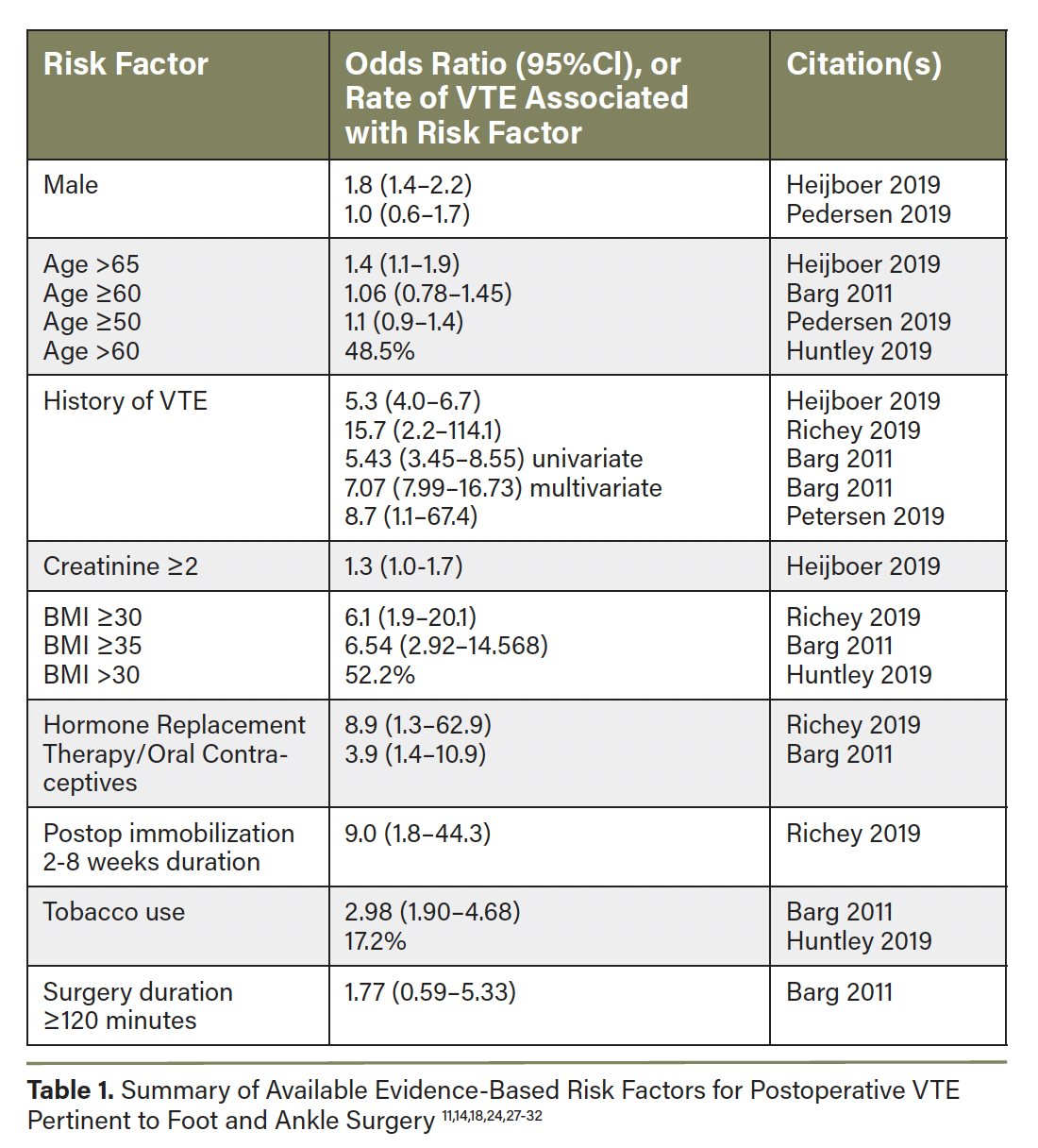
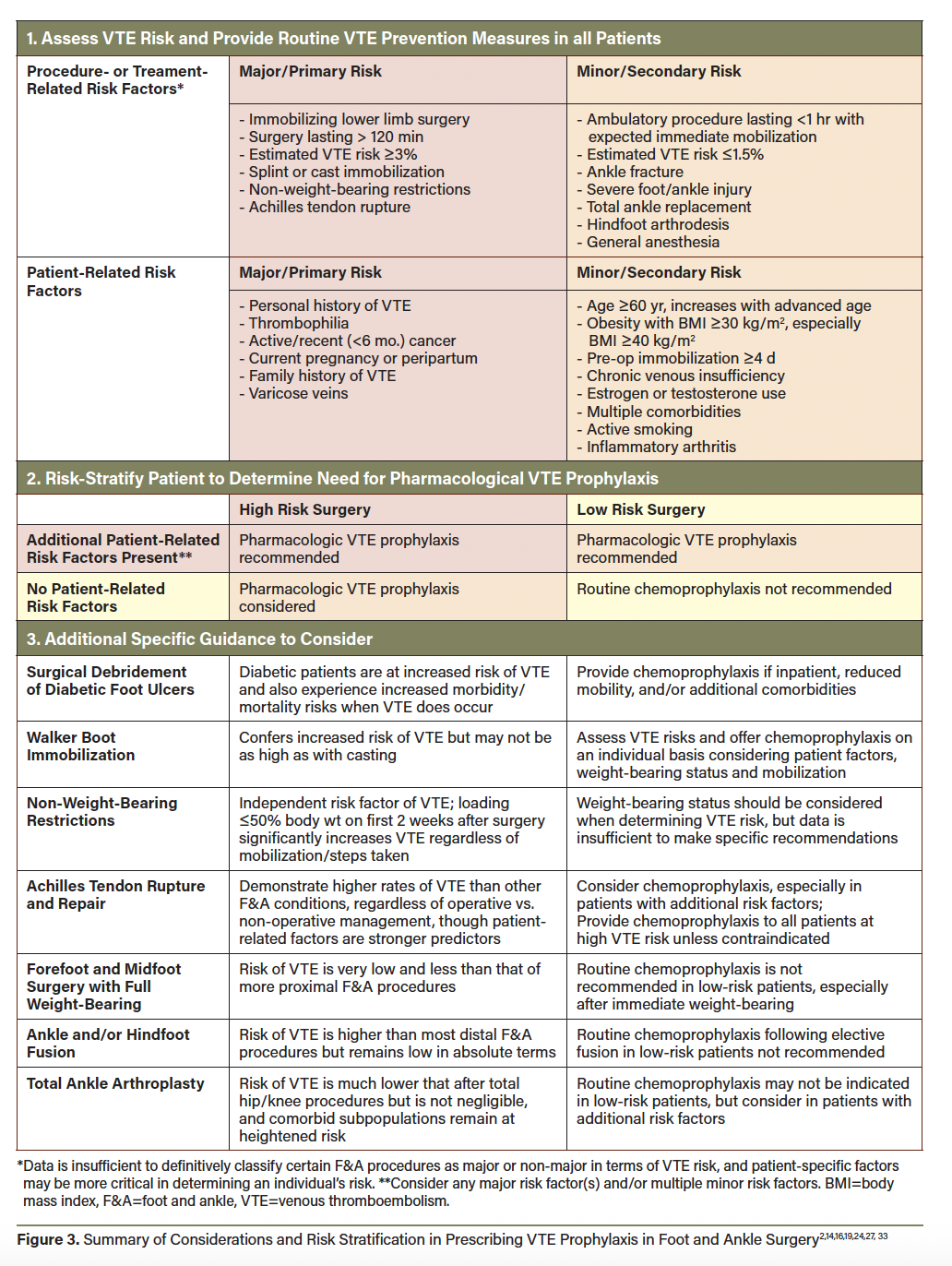
No singular risk stratification scheme has been prospectively validated for informing VTE chemoprophylaxis after orthopedic or foot and ankle surgery. However, certain patient and surgery-specific factors confer VTE risk and should be considered when determining if chemoprophylaxis is likely to benefit a particular patient (Table 1).11,14,18,24,27-32 Some guidelines support an integrated risk stratification such as that summarized in Figure 3 2,14,16,19,24,27,33 to decide when to prescribe pharmacologic VTE prophylaxis after foot and ankle procedures/injuries. The Leiden-Thrombosis Risk Prediction (L-TRiP[cast]) score and the Trauma, Immobilization, Patient characteristics (TIP) score are proposed VTE risk assessment models in patients requiring lower leg casting.34-36
Pharmacologic Agent Selection
Multiple antithrombotic agents have demonstrated efficacy in reducing VTE after major orthopedic surgery of the hip and knee, with data being more sparse in foot and ankle surgery. Current guidelines from the American College of Chest Physicians and from the American Society of Hematology support the use of LMWH (eg, enoxaparin), direct oral anticoagulants (DOACs, including apixaban, rivaroxaban, edoxaban, and dabigatran), or aspirin for VTE prophylaxis after major orthopedic surgery, in combination with nonpharmacologic mechanisms.7,37 The European guidelines on perioperative VTE prophylaxis in ambulatory/ fast-track surgical procedures suggest a risk-stratified approach considering patient- and procedure-related factors. Patients undergoing high-risk surgeries, including immobilizing lower limb surgery and/or surgery lasting >120 mins, as well as high-risk patients undergoing any ambulatory surgery, are recommended to receive pharmacologic VTE prophylaxis in addition to general thromboprophylaxis measures.19 The DOACs, LMWH, and aspirin are again all supported modalities in orthopedic surgery.19,38 Previous guidance statements from foot and ankle surgery organizations are limited based on available data, providing no clear recommendations on agent selection.1,2 The International Consensus Meeting on Venous Thromboembolism (ICM-VTE) foot and ankle practice guidelines provide an updated literature review and recommendations on various aspects of VTE prophylaxis without advising on agent selection.24
Few randomized controlled trials (RCTs) in modern foot and ankle surgical populations exist to inform chemoprophylaxis selection. The 2020 international PRONOMOS trial randomized patients receiving nonmajor lower limb orthopedic surgery at risk for VTE to either rivaroxaban or enoxaparin prophylaxis postoperatively until the end of immobilization.39 The study population included patients undergoing Achilles’ tendon repair, ankle fracture repair, ankle or hindfoot arthrodesis, ankle ligament repair, and other elective lower limb procedures that had indication for at least 2 weeks’ thromboprophylaxis. The primary composite outcome of symptomatic DVT, PE, or death during treatment or asymptomatic proximal DVT at end of treatment occurred in 0.2% of rivaroxaban-treated patients vs. 1.1% of enoxaparin-treated patients (RR 0.25, 95%CI 0.09-0.75, P<0.001 for noninferiority, P=0.01 for superiority, n=3301).39 This difference was primarily driven by lower rates of symptomatic DVT (0.2% vs. 0.6%) and asymptomatic proximal DVT (0.1% vs 0.4%) in the rivaroxaban arm. Major or clinically relevant nonmajor bleeding events occurred at 1.1% in the rivaroxaban arm versus 1.0% in the enoxaparin arm (RR 1.04, 95%CI 0.55-2.00, P=0.89), for an estimated net clinical harm rate of 0.8% versus 1.8% including both VTE and major bleeding events.39 Significant limitations of this study include premature halting of the trial before power was met, exclusion of >100 randomized patients from each study arm from the primary “intention-to-treat” analysis due to premature study withdrawal or missing data, and industry sponsorship. Overall, this study highlights that both VTE and bleeding complications are rare with these agents after nonmajor orthopedic surgery, and both represent reasonable options for chemoprophylaxis.
An investigator-initiated RCT of orthopedic trauma patients with operative pelvic or lower extremity fractures, with 36% of the study population comprising foot and ankle injuries, also randomized patients to rivaroxaban vs. enoxaparin prophylaxis postoperatively.40 This study found very low rates of thrombotic and bleeding events with no significant differences observed between agents, though patient satisfaction was significantly higher in the rivaroxaban group.40 A recent meta-analysis of RCTs assessing the effectiveness and safety of rivaroxaban versus LMWH in nonmajor orthopedic surgery found no significant differences in VTE or bleeding events (5 RCTs, N=5101), further supporting the appropriateness of either modality.41
In the face of limited guideline recommendations and comparative data specific to foot and ankle surgery, the choice of chemoprophylactic agent is also informed by data from major orthopedic surgery, medication properties, and patient-specific factors (Table 2).39,41-46 Many landmark RCTs in total hip and knee arthroplasty established the efficacy and safety of DOACs like rivaroxaban and apixaban as compared to LMWH, and they are supported in preference to LMWH by current guidelines.37,43 Rivaroxaban prophylaxis significantly reduced symptomatic VTE compared to enoxaparin with a small increase in clinically relevant bleeding, whereas apixaban significantly reduced VTE without increased bleeding events (and some bleeding events were lower with apixaban in some RCTs).44,47,48 While these data may suggest apixaban has the most favorable risk-benefit profile among the DOACs, it is important to acknowledge that only indirect comparisons are available to inform this assessment since prospective RCTs comparing DOACs remain elusive.43
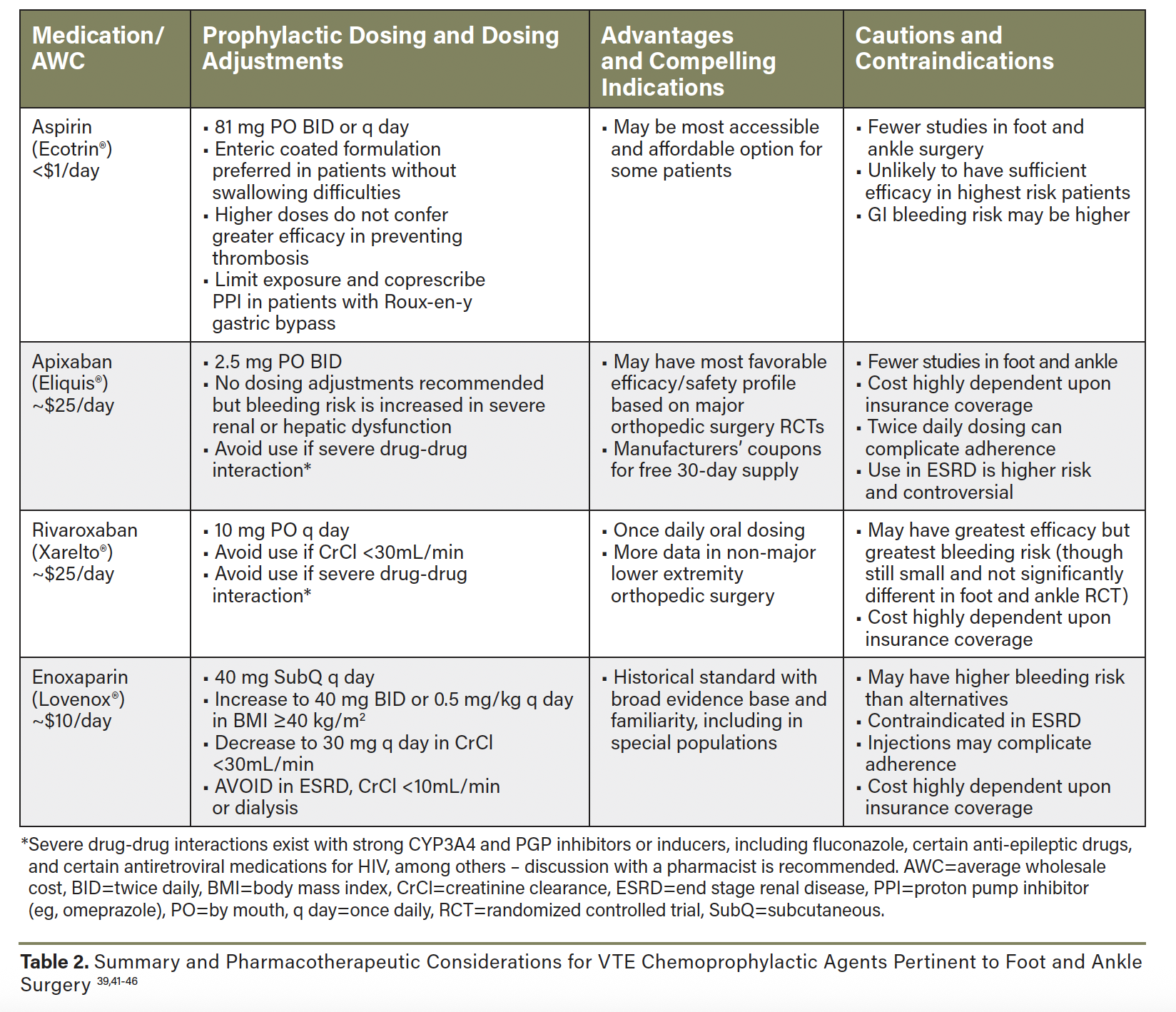
The role of aspirin as a more accessible and appreciably effective thromboprophylactic agent continues to expand in the orthopedic literature,49 with multiple recent RCTs providing high-quality comparative efficacy data. While the large multicenter CRISTAL trial of adult hip or knee arthroplasty patients saw significantly lower rates of symptomatic VTE with enoxaparin vs. aspirin (1.8% vs. 3.5%, P=0.007, n=9203), this difference was primarily driven by lower rates of distal DVT,50 which are of waning clinical significance per current management guidelines.7 Major bleeding events were rare (0.3–0.4%) and not significantly different between enoxaparin and aspirin.50 The recent PREVENT CLOT trial in patients with an operative extremity fracture or any hip/pelvic fracture similarly suggested significantly lower rates of symptomatic DVT with enoxaparin versus aspirin, though rates were low overall (1.7% vs. 2.5%) and bleeding was not significantly different between groups.51 Data for aspirin as a sole prophylactic agent in foot and ankle surgery has been of low quality and conflicting, though it appears to be a reasonable option in patients without major VTE risk factors.13,52 In aggregate, the prior literature, experience, and accessibility of aspirin make it a reasonable and attractive option for lower risk patients, though it has demonstrated inferior efficacy to anticoagulant prophylaxis in high risk patients.50 Ongoing RCTs will compare aspirin to DOACs for postoperative chemoprophylaxis.53,54
Beyond clinical data, the choice of prophylactic agent must consider patient adherence and affordability in order to be successful. Some patients may be averse to injections, while others will adhere more significantly to once-daily versus twice-daily dosing regimens. Insured patients will often see significantly greater affordability with one DOAC over another based on insurance formulary preferences, whereas un- or under-insured patients may benefit from manufacturer coupons to access free trials of DOAC that could be used to cover the prophylactic course. We find that enoxaparin injections may be completely covered, but only up to a certain quantity/days supplied. Aspirin likely remains the most accessible option if cost barriers arise.
Timing and Duration of VTE Chemoprophylaxis
The optimal time to initiate postoperative VTE chemoprophylaxis, while not completely elucidated, is typically between 6–24 hours after case end, assuming satisfactory hemostasis and acceptable bleeding risk.14,22 One can achieve this by ordering a chemoprophylactic agent beginning the day/evening of surgery, or beginning the morning of postop day one for surgical cases that complete late in the day. While concerns with bleeding risk can arise with this strategy, it is important to recall that postoperative VTE risk is greatest immediately after surgery when the factors of Virchow’s triad are most significant. Available data in major orthopedic surgery suggest approximately 35% of postoperative VTEs present in the first week following surgery, with about 20% occurring in the second postop week, 10% in the third, 8% in the fourth, and the remaining 27% in the second and third months.55-57
The optimal duration of VTE chemoprophylaxis after foot and ankle surgery is unestablished and likely varies widely depending on the procedure and postoperative weight-bearing status. Some recommend therapy extend for the length of immobilization and/or reduced weight-bearing.2,14,42 Guidelines in orthopedic surgery recommend durations of 28–35 days.19,22 Multiple studies suggest that the entirety of this duration need not include an anticoagulant, however, with de-escalation to aspirin from an LMWH or DOAC after the initial prophylactic anticoagulant course achieving comparable outcomes to full anticoagulant courses.58-62 This hybrid or “step down” approach to VTE chemoprophylaxis capitalizes on the front-loaded distribution of postoperative VTE and can spare excess exposure to potentially costly anticoagulant agents.
Patient Education and Monitoring
Patient education for recognition of signs and symptoms of VTE is crucial. At our institution, during the initial preoperative consultation, the patient receives education on the possibility of VTE as a potential life-threatening complication following foot and ankle surgery. The patient is instructed to monitor for signs of possible DVT or PE, including increased edema and erythema to the operative extremity, as well as increased pain to the calf and more threatening signs such as shortness of breath or chest pain.
Postoperatively, clinical pharmacists assist with comprehensive discharge medication optimization. This includes ensuring appropriate, accessible VTE prophylaxis prescriptions and counseling patients at bedside prior to discharge on their specific form of VTE prophylaxis. Key counseling points include how to take the medication (including education on appropriate administration technique for LMWH injections), importance of adherence for the duration of the intended treatment course, how to respond to symptoms of VTE or medication side effects, and any implications of the VTE prophylaxis regimen for other chronic medications. The pharmacist proactively assesses barriers to successful completion of the full VTE prophylaxis course and mitigates these in concert with the surgical team. This additional multidisciplinary component to patient care allows for continuity and coordination across phases of care and surgical providers to target improved medication adherence and optimal patient outcomes.63
In Summary
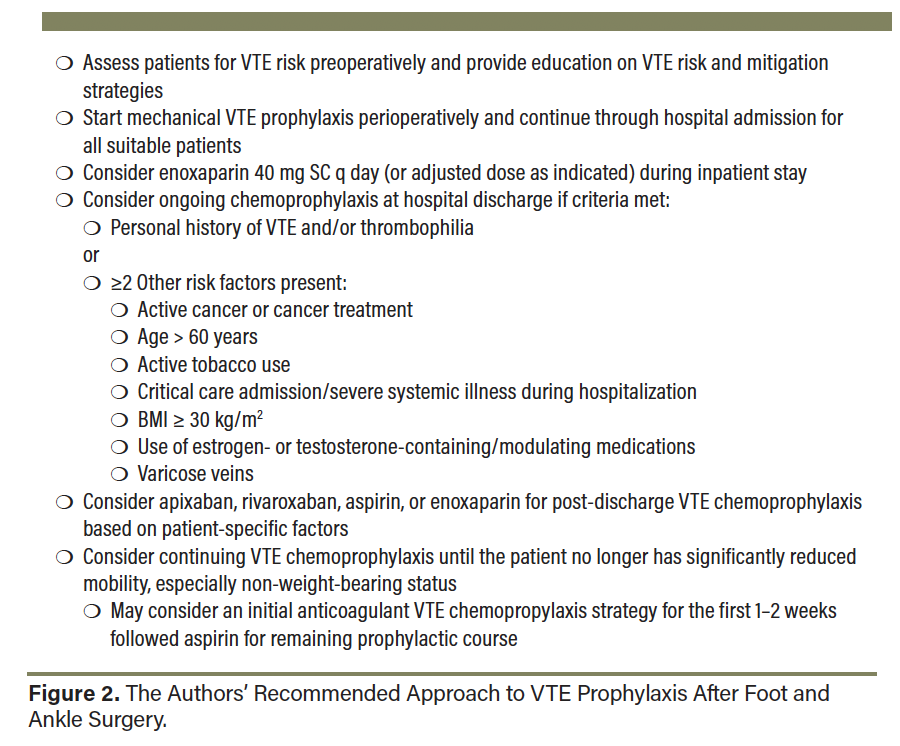
Overall, though both DVT and PE have a low incidence following foot and ankle surgery, these complications are potentially life-threatening and may cause significant morbidity to patients. Considering the relatively limited amount of high-quality data to guide VTE prevention in foot and ankle surgery, providers are left with the challenge of appropriately selecting both mechanical and chemical VTE prophylactic agents. Figure 2 shows our recommended approach to VTE prophylaxis following foot and ankle surgery. Further data such as prospective studies and RCTs are needed to assist in delineating guidelines for VTE prophylaxis in foot and ankle surgery.
1. AOFAS. Position Statement: The use of venous thromboembolic disease prophylaxis in foot and ankle surgery. The American Orthopaedic Foot & Ankle Society; February 2020. Available from: https://www.aofas.org/docs/default-source/research-and-policy/position-statements/vted-prophylaxis-in-foot-and-ankle-surgery-position-statement.pdf?sfvrsn=21490028_2
2. Fleischer AE, Abicht BP, Baker JR, Boffeli TJ, Jupiter DC, Schade VL. American College of Foot and Ankle Surgeons’ clinical consensus statement: risk, prevention, and diagnosis of venous thromboembolism disease in foot and ankle surgery and injuries requiring immobilization. J Foot Ankle Surg. 2015;54(3):497-507. doi:10.1053/j.jfas.2015.02.022
3. Kushner A, West WP, Khan SMZ, Pillarisetty LS. Virchow Triad. StatPearls Publishing; 2022.
4. McLendon K, Goyal A, Attia M. Deep venous thrombosis risk factors. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2025.
5. Jameson SS, Augustine A, James P, et al. Venous thromboembolic events following foot and ankle surgery in the English National Health Service. J Bone Joint Surg Br. 2011;93(4):490-497. doi:10.1302/0301-620X.93B4.25731
6. Solis G, Saxby T. Incidence of DVT following surgery of the foot and ankle. Foot Ankle Int. 2002;23(5):411-414. doi:10.1177/107110070202300507
7. Stevens SM, Woller SC, Baumann Kreuziger L, et al. Antithrombotic therapy for VTE disease. Chest. 2024. doi:10.1016/j.chest.2024.03.003
8. Heijboer RRO, Lubberts B, Guss D, et al. Venous thromboembolism and bleeding adverse events in lower leg, ankle, and foot orthopaedic surgery with and without anticoagulants. J Bone Joint Surg Am. 2019;101(6):539-546. doi:10.2106/JBJS.18.00346
9. Shibuya N, Zimmer C, Jupiter DC. Venous thromboembolism in foot and ankle trauma. Clin Podiatr Med Surg. 2024;41(4):607-617. doi:10.1016/j.cpm.2024.01.012
10. Selby R, Geerts WH, Kreder HJ, et al. Symptomatic venous thromboembolism uncommon without thromboprophylaxis after isolated lower-limb fracture. J Bone Joint Surg Am. 2014;96(12):e83. doi:10.2106/jbjs.m.00236
11. Wahlsten LR, Eckardt H, Lyngbæk S, et al. Symptomatic venous thromboembolism following fractures distal to the knee: a nationwide Danish cohort study. J Bone Joint Surg Am. 2015;97(6):470-477. doi:10.2106/JBJS.N.00307
12. Nemeth B, Cannegieter SC. Venous thrombosis following lower-leg cast immobilization and knee arthroscopy: From a population-based approach to individualized therapy. Thromb Res. 2019;174:62-75. doi:10.1016/j.thromres.2018.11.030
13. Griffiths JT, Matthews L, Pearce CJ, Calder JDF. Incidence of venous thromboembolism in elective foot and ankle surgery with and without aspirin prophylaxis. J Bone Joint Surg Br. 2012;94(2):210-214. doi:10.1302/0301-620X.94B2.27579
14. Calder JDF, Freeman R, Domeij-Arverud E, van Dijk CN, Ackermann PW. Meta-analysis and suggested guidelines for prevention of venous thromboembolism (VTE) in foot and ankle surgery. Knee Surg Sports Traumatol Arthrosc. 2016;24(4):1409-1420. doi:10.1007/s00167-015-3976-y
15. Martinazzi BJ, Kirchner GJ, Stauch CM, et al. Cost-effective modeling of thromboembolic chemoprophylaxis for total ankle arthroplasty. Foot Ankle Int. 2022;43(11):1379-1384. doi:10.1177/10711007221112922
16. AOFAS. Position Statement: Management of Acute Achilles Tendon Ruptures. American Orthopaedic Foot & Ankle Society; 2024. Available from: https://www.aofas.org/docs/default-source/research-and-policy/position-statements/positionstatement_acuteachillesruptures_nov2024.pdf?sfvrsn=7c175c5a_3
17. Barfod KW, Nielsen EG, Olsen BH, et al. Risk of deep vein thrombosis after acute Achilles tendon rupture: a secondary analysis of a randomized controlled trial comparing early controlled motion of the ankle versus immobilization. Orthop J Sports Med. 2020;8(4):2325967120915909. doi:10.1177/2325967120915909
18. Pedersen MH, Wahlsten LR, Grønborg H, et al. Symptomatic venous thromboembolism after Achilles tendon rupture: a nationwide Danish cohort study of 28,546 patients with Achilles tendon rupture. Am J Sports Med. 2019;47(13):3229-3237. doi:10.1177/0363546519876054
19. Jørgensen CC, Llau J, Jenny J-Y, Albaladejo P. European guidelines on peri-operative venous thromboembolism prophylaxis: first update. Chapter 3: Day surgery and fast-track surgery. Eur J Anaesthesiol. 2024;41(9):577-581. doi:10.1097/EJA.0000000000002010
20. Aufwerber S, Heijne A, Edman G, Grävare Silbernagel K, Ackermann PW. Early mobilization does not reduce the risk of deep venous thrombosis after Achilles tendon rupture: a randomized controlled trial. Knee Surg Sports Traumatol Arthrosc. 2020;28(1):312-319. doi:10.1007/s00167-019-05767-x
21. Li Y, Guan X-H, Wang R, et al. Active ankle movements prevent formation of lower-extremity deep venous thrombosis after orthopedic surgery. Med Sci Monit. 2016;22:3169-3176. doi:10.12659/msm.896911
22. Falck-Ytter Y, Francis CW, Johanson NA, et al. Prevention of VTE in orthopedic surgery patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2_suppl):e278S-e325S. doi:10.1378/chest.11-2404
23. National Guideline Centre (UK). Venous thromboembolism in over 16s: reducing the risk of hospital-acquired deep vein thrombosis or pulmonary embolism. London: National Institute for Health and Care Excellence (NICE); 2018.
24. The ICM-VTE Foot & Ankle Delegates. Recommendations from the ICM-VTE: Foot & Ankle. J Bone Joint Surg Am. 2022;104(2):163-175. doi:10.2106/JBJS.21.01439
25. Zee AA, van Lieshout K, van der Heide M, Janssen L, Janzing HM. Low molecular weight heparin for prevention of venous thromboembolism in patients with lower-limb immobilization. Cochrane Database Syst Rev. 2017;8:CD006681. doi:10.1002/14651858.CD006681.pub4
26. Bikdeli B, Visvanathan R, Jimenez D, et al. Use of prophylaxis for prevention of venous thromboembolism in patients with isolated foot or ankle surgery: a systematic review and meta-analysis. Thromb Haemost. 2019;119(10):1686-1694. doi:10.1055/s-0039-1693464
27. Weisman MHS, Holmes JR, Irwin TA, Talusan PG. Venous thromboembolic prophylaxis in foot and ankle surgery: a review of current literature and practice. Foot Ankle Spec. 2017;10(4):343-351. doi:10.1177/1938640017692417
28. Heijboer RRO, Lubberts B, Guss D, Johnson AH, DiGiovanni CW. Incidence and risk factors associated with venous thromboembolism after orthopaedic below-knee surgery. J Am Acad Orthop Surg. 2019;27(14):e482-e490. doi:10.5435/JAAOS-D-17-00787
29. Huntley SR, Abyar E, Lehtonen EJ, et al. Incidence of and risk factors for venous thromboembolism after foot and ankle surgery. Foot Ankle Spec. 2019;12(3):218-227. doi:10.1177/1938640018769740
30. Richey JM, Ritterman Weintraub ML, Schuberth JM. Incidence and risk factors of symptomatic venous thromboembolism following foot and ankle surgery. Foot Ankle Int. 2019;40(1):98-104. doi:10.1177/1071100718794851
31. Nemeth B, Timp JF, van Hylckama Vlieg A, Rosendaal FR, Cannegieter SC. High risk of recurrent venous thrombosis in patients with lower-leg cast immobilization. J Thromb Haemost. 2018;16(11):2218-2222. doi:10.1111/jth.14278
32. Barg A, Henninger HB, Hintermann B. Risk factors for symptomatic deep-vein thrombosis in patients after total ankle replacement who received routine chemical thromboprophylaxis. J Bone Joint Surg Br. 2011;93(7):921-927. doi:10.1302/0301-620X.93B7.26257
33. Zhou S, Zheng H, Cao M, et al. Comparative efficacy of cast immobilization versus removable braces in patients with ankle fractures: a systematic review and meta-analysis. BMC Musculoskelet Disord. 2025;26:243. doi:10.1186/s12891-025-08451-z
34. Nemeth B, van Adrichem R, Nelissen R, le Cessie S, Cannegieter SC. Individualized thromboprophylaxis in patients with lower-leg cast immobilization: a validation and subgroup analysis in the POT-CAST trial. Thromb Haemost. 2019;119(9):1508-1516. doi:10.1055/s-0039-1693410
35. Douillet D, Nemeth B, Penaloza A, et al. Venous thromboembolism risk stratification for patients with lower limb trauma and cast or brace immobilization. PLoS One. 2019;14(6):e0217748. doi:10.1371/journal.pone.0217748
36. Douillet D, Penaloza A, Viglino D, et al. Targeted prophylactic anticoagulation based on the TRiP(cast) score in patients with lower limb immobilisation: a multicentre, stepped wedge, randomised implementation trial. Lancet. 2024;403(10330):1051-1060. doi:10.1016/s0140-6736(23)02369-3
37. Anderson DR, Morgano GP, Bennett C, et al. American Society of Hematology 2019 guidelines for management of venous thromboembolism: prevention of venous thromboembolism in surgical hospitalized patients. Blood Adv. 2019;3(23):3898-3944. doi:10.1182/bloodadvances.2019000975
38. Jenny J-Y, Godier A, Heim C, et al. European guidelines on peri-operative venous thromboembolism prophylaxis: first update. Chapter 13: Nonambulatory orthopaedic surgery. Eur J Anaesthesiol. 2024;41(10):622-626. doi:10.1097/EJA.0000000000002020
39. Samama CM, Laporte S, Rosencher N, et al. Rivaroxaban or enoxaparin in nonmajor orthopedic surgery. N Engl J Med. 2020;382(20):1916-1925. doi:10.1056/NEJMoa1913808
40. John MP 2nd, Streufert BD, Downes K, Chase CB, Mir HR. A prospective randomized controlled trial comparing enoxaparin and rivaroxaban for venous thromboembolism prophylaxis in orthopaedic trauma. J Orthop Trauma. 2022;36(11):615-622. doi:10.1097/BOT.0000000000002454
41. Zhu L, Zhu B, Bing P, et al. Effectiveness and safety of rivaroxaban or low-molecular-weight heparin in non-major orthopedic surgery: a meta-analysis of randomized controlled trials. J Orthop Surg Res. 2024;19:609. doi:10.1186/s13018-024-05087-y
42. Kahn SR, Shivakumar S. What’s new in VTE risk and prevention in orthopedic surgery. Res Pract Thromb Haemost. 2020;4(3):366-376. doi:10.1002/rth2.12323
43. Tun HN, Kyaw MT, Rafflenbeul E, Suástegui XL. Role of direct oral anticoagulants for post-operative venous thromboembolism prophylaxis. Eur Cardiol. 2022;17:e11. doi:10.15420/ecr.2021.55
44. Mahan CE, Kaatz S. Performance of new anticoagulants for thromboprophylaxis in patients undergoing hip and knee replacement surgery. Pharmacotherapy. 2012;32(11):1036-1048. doi:10.1002/phar.1133
45. Lexi-Drugs. UpToDate Lexidrug [internet database]. UpToDate Inc. https://online.lexi.com. Accessed June 2, 2025.
46. Garcia DA, Baglin TP, Weitz JI, Samama MM. Parenteral anticoagulants. Chest. 2012;141(2_suppl):e24S-e43S. doi:10.1378/chest.11-2291
47. Turpie AGG, Lassen MR, Eriksson BI, et al. Rivaroxaban for the prevention of venous thromboembolism after hip or knee arthroplasty: pooled analysis of four studies. Thromb Haemost. 2011;105(3):444-453. doi:10.1160/TH10-09-0601
48. Raskob GE, Gallus AS, Pineo GF, et al. Apixaban versus enoxaparin for thromboprophylaxis after hip or knee replacement: pooled analysis of major venous thromboembolism and bleeding in 8464 patients from the ADVANCE-2 and ADVANCE-3 trials. J Bone Joint Surg Br. 2012;94(2):257-264. doi:10.1302/0301-620X.94B2.27850
49. Parvizi J, Ceylan HH, Kucukdurmaz F, et al. Venous thromboembolism following hip and knee arthroplasty: the role of aspirin. J Bone Joint Surg Am. 2017;99(11):961-972. doi:10.2106/JBJS.16.01253
50. CRISTAL Study Group, Sidhu VS, Kelly T-L, et al. Effect of aspirin vs enoxaparin on symptomatic venous thromboembolism in patients undergoing hip or knee arthroplasty: the CRISTAL randomized trial. JAMA. 2022;328(8):719-727. doi:10.1001/jama.2022.13416
51. Major Extremity Trauma Research Consortium (METRC), O’Toole RV, Stein DM, et al. Aspirin or low-molecular-weight heparin for thromboprophylaxis after a fracture. N Engl J Med. 2023;388(3):203-213. doi:10.1056/NEJMoa2205973
52. Sidhu V, Adams AJ, Kachooei AR, et al. Routine postoperative aspirin for VTE chemoprophylaxis in primary total ankle arthroplasty. Foot Ankle Orthop. 2022;7:2473011421S0094. doi:10.1177/2473011421s00940
53. Shivakumar S. VTE Prevention Following Total Hip and Knee Arthroplasty (EPCATIII). Clinical Trials ID NCT04075240. Updated Feb. 7, 2024.
54. Dartmouth-Hitchcock Medical Center. Comparative Effectiveness of Pulmonary Embolism Prevention After Hip and Knee Replacement (PEPPER). Clinical Trials ID NCT02810704. Updated Jan. 22, 2025.
55. Petersen PB, Kehlet H, Jørgensen CC; Lundbeck Foundation Centre for Fast-track Hip and Knee Replacement Collaborative Group. Safety of in-hospital only thromboprophylaxis after fast-track total hip and knee arthroplasty: a prospective follow-up study in 17,582 procedures. Thromb Haemost. 2018;118(12):2152-2161. doi:10.1055/s-0038-1675641
56. Jørgensen CC, Jacobsen MK, Soeballe K, et al. Thromboprophylaxis only during hospitalisation in fast-track hip and knee arthroplasty, a prospective cohort study. BMJ Open. 2013;3(12):e003965. doi:10.1136/bmjopen-2013-003965
57. Pedersen AB, Andersen IT, Overgaard S, et al. Optimal duration of anticoagulant thromboprophylaxis in total hip arthroplasty: new evidence in 55,540 patients with osteoarthritis from the Nordic Arthroplasty Register Association (NARA) group. Acta Orthop. 2019;90(4):298-305. doi:10.1080/17453674.2019.1611215
58. Anderson DR, Dunbar MJ, Bohm ER, et al. Aspirin versus low-molecular-weight heparin for extended venous thromboembolism prophylaxis after total hip arthroplasty: a randomized trial. Ann Intern Med. 2013;158(11):800-806. doi:10.7326/0003-4819-158-11-201306040-00004
59. Anderson DR, Dunbar M, Murnaghan J, et al. Aspirin or rivaroxaban for VTE prophylaxis after hip or knee arthroplasty. N Engl J Med. 2018;378(8):699-707. doi:10.1056/NEJMoa1712746
60. Peng H-M, Chen X, Wang Y-O, et al. Risk-stratified venous thromboembolism prophylaxis after total joint arthroplasty: low molecular weight heparins and sequential aspirin vs aggressive chemoprophylaxis. Orthop Surg. 2021;13(1):260-266. doi:10.1111/os.12926
61. Mihara M, Tamaki Y, Nakura N, et al. Clinical efficacy of risk-stratified prophylaxis with low-dose aspirin for the management of symptomatic venous thromboembolism after total hip arthroplasty. J Orthop Sci. 2020;25(1):156-160. doi:10.1016/j.jos.2019.02.009
62. Hyland SJ, Fada MJ, Secic M, et al. Risk-stratified venous thromboembolism chemoprophylaxis after total joint arthroplasty: evaluation of an institutional approach. J Clin Med. 2025;14(2):366. doi:10.3390/jcm14020366
63. Hyland SJ, Kramer BJ, Fada RA, Lucki MM. Clinical pharmacist service associated with improved outcomes and cost savings in total joint arthroplasty. J Arthroplasty. 2020;35(9):2307-2317.e1. doi:10.1016/j.arth.2020.04.022











