Management of Flush In-Stent Occlusion in the Superficial Femoral Artery Using the Detour Percutaneous Bypass System
Abstract
Percutaneous bypass is being used more frequently for patients with long-segment superficial femoral artery (SFA) occlusions. However, to our knowledge, this technique has not been previously described in patients with occluded SFA stents extending to the common femoral artery bifurcation. This case report describes 2 different techniques for using percutaneous bypass in patients with prior SFA stent occlusions extending to the common femoral bifurcation.
J CRIT LIMB ISCHEM 2025:5(4):E44-E51. doi: 10.25270/jcli/CLIG-2500007
Key words: femoropopliteal disease, peripheral arterial disease, endovascular therapy, superficial femoral artery, stent occlusion, lower extremity bypass, percutaneous transfemoral arterial bypass
Superficial femoral artery (SFA) disease represents one of the most commonly involved sites in patients with peripheral arterial disease (PAD).1 The management of SFA disease has evolved significantly over the past 2 decades, transitioning from primarily a surgical approach to endovascular-first strategies.1 However, the SFA presents unique challenges for endovascular techniques.1 The vessel’s length, mobility, and exposure to mechanical stress contribute to higher rates of restenosis, occlusion, and stent fracture.1 Open surgical interventions that include bypass grafting have good long-term outcomes but increase the risk of perioperative complications.2 Shorter lesions (Trans-Atlantic Inter-Society Consensus [TASC] A and B) respond well to a variety of different endovascular techniques, including balloon angioplasty, self-expandable stenting, antirestenotic drug delivery, and intravascular lithotripsy.3 Primary patency rates for endovascular interventions typically range from 60% to 80% at 1 year post-procedure, depending on lesion characteristics and intervention type.4
For more extensive or widespread arterial disease (TASC C and D), endovascular treatment can be challenging and often results in poor patency rates with a need for more frequent reinterventions.1,5,6 Until recently, open surgery was the only other treatment option in patients with complex SFA disease, especially following recurrence after endovascular recanalization techniques. More recently, endovascular percutaneous transfemoral arterial bypass (PTAB) is being utilized as a minimally invasive treatment option for patients with extensive, complex SFA pathology.7-9 PTAB with the Detour system (Endologix) includes the Endocross crossing device and Torus stent graft to restore blood flow to the lower leg by creating a bypass route through the femoral vein that circumvents the blocked SFA.7 This minimally invasive bypass technique decreases the likelihood of surgical site infections and other adverse local and systemic events that typically occur with more invasive traditional open bypass procedures. This case report describes the technical aspects of using PTAB in 2 patients with occluded SFA stents located in the proximal landing zone.
Case Report (Thru-Stent Technique)
Initial Presentation
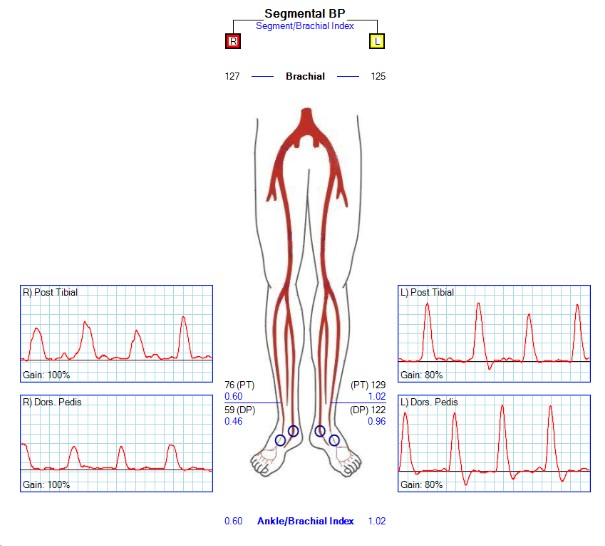
A 53-year-old woman presented with lifestyle-limiting right leg claudication. She was not a current smoker and had a history of an initial right SFA angioplasty that subsequently occluded, requiring a SFA stent (EverFlex, Medtronic). The SFA stent then reoccluded, requiring re-angioplasty and extension of the stent to the SFA/common femoral junction. She now presented with reocclusion (X3) and short-distance claudication. The patient had a family history of deep vein thrombosis (DVT). Coagulation workup did not identify any form of hypercoagulability. Vein mapping demonstrated an average diameter of 1.28 mm for both ipsilateral and contralateral greater saphenous veins. Preoperative ankle-brachial index (ABI) was 0.6 (Figure 1). Treatment options, including conservative management with exercise therapy, synthetic bypass, recanalization of occluded SFA stents, and PTAB, were discussed with the patient, who opted for the PTAB procedure.
Procedural Overview
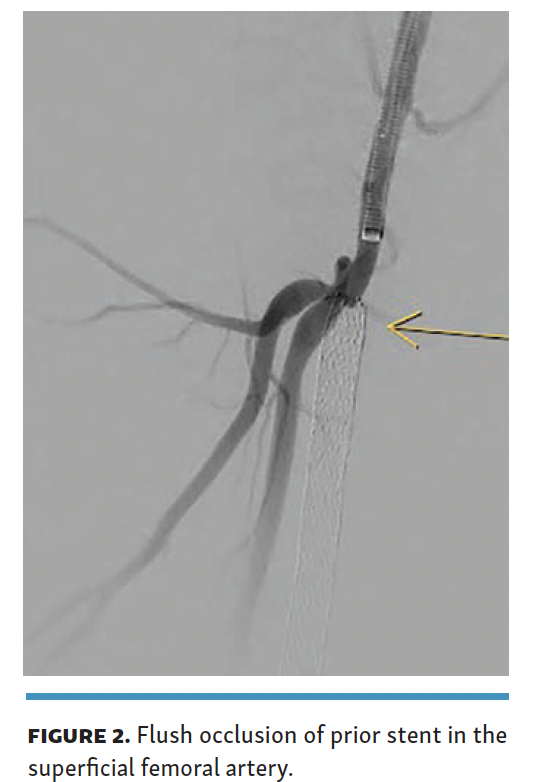 For the procedure, 8F arterial access was obtained from the contralateral side with ipsilateral posterior tibial venous access (6F, 25-cm sheath). Diagnostic angiogram demonstrated a flush occlusion of the prior SFA stent with no proximal landing zone in the SFA (Figure 2).
For the procedure, 8F arterial access was obtained from the contralateral side with ipsilateral posterior tibial venous access (6F, 25-cm sheath). Diagnostic angiogram demonstrated a flush occlusion of the prior SFA stent with no proximal landing zone in the SFA (Figure 2).
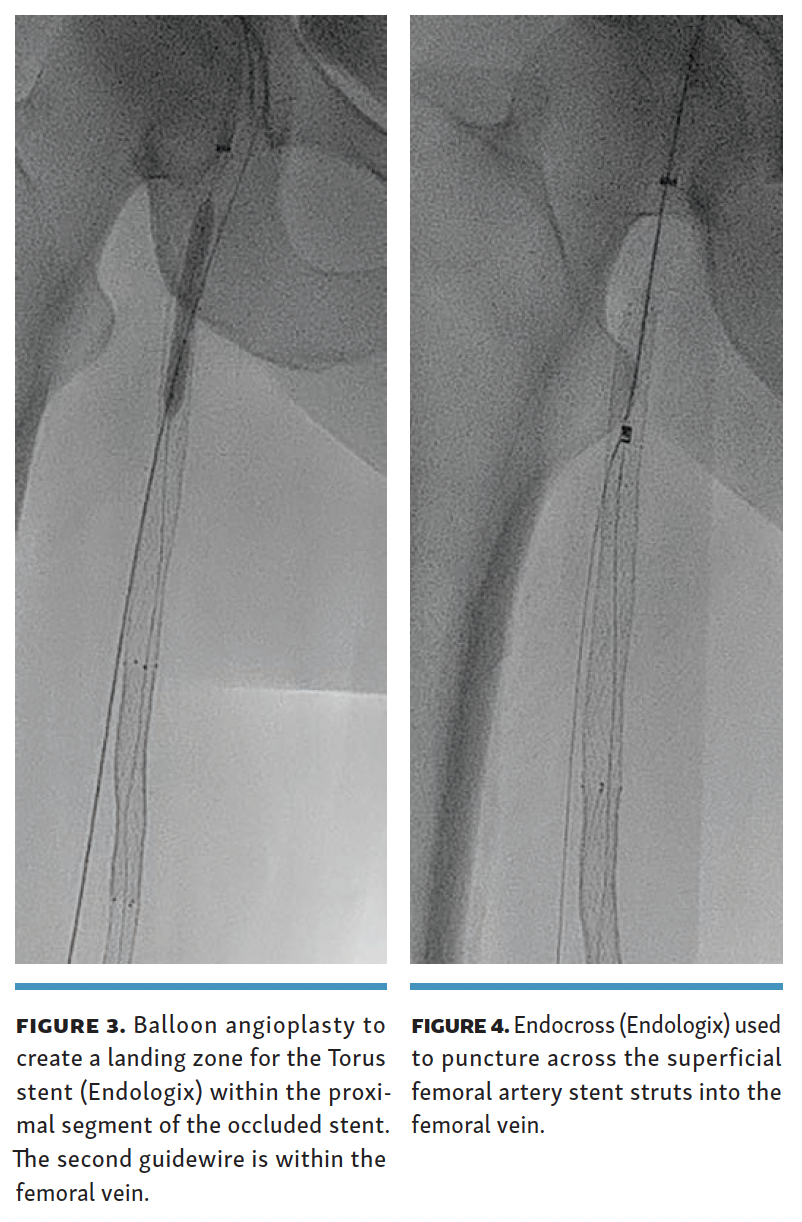
A 0.014-inch Glidewire Advantage Track wire (Terumo) was used to reenter the occluded SFA stent with the help of a Quick-Cross support catheter (Philips), followed by a 4-mm balloon angioplasty of the occluded proximal SFA stent (Figure 3). The Endocross device was then placed in the recanalized proximal SFA stent, and needle entry was obtained into the femoral vein through the stent struts (Figure 4) 5 cm below the proximal end of the occluded SFA stent.
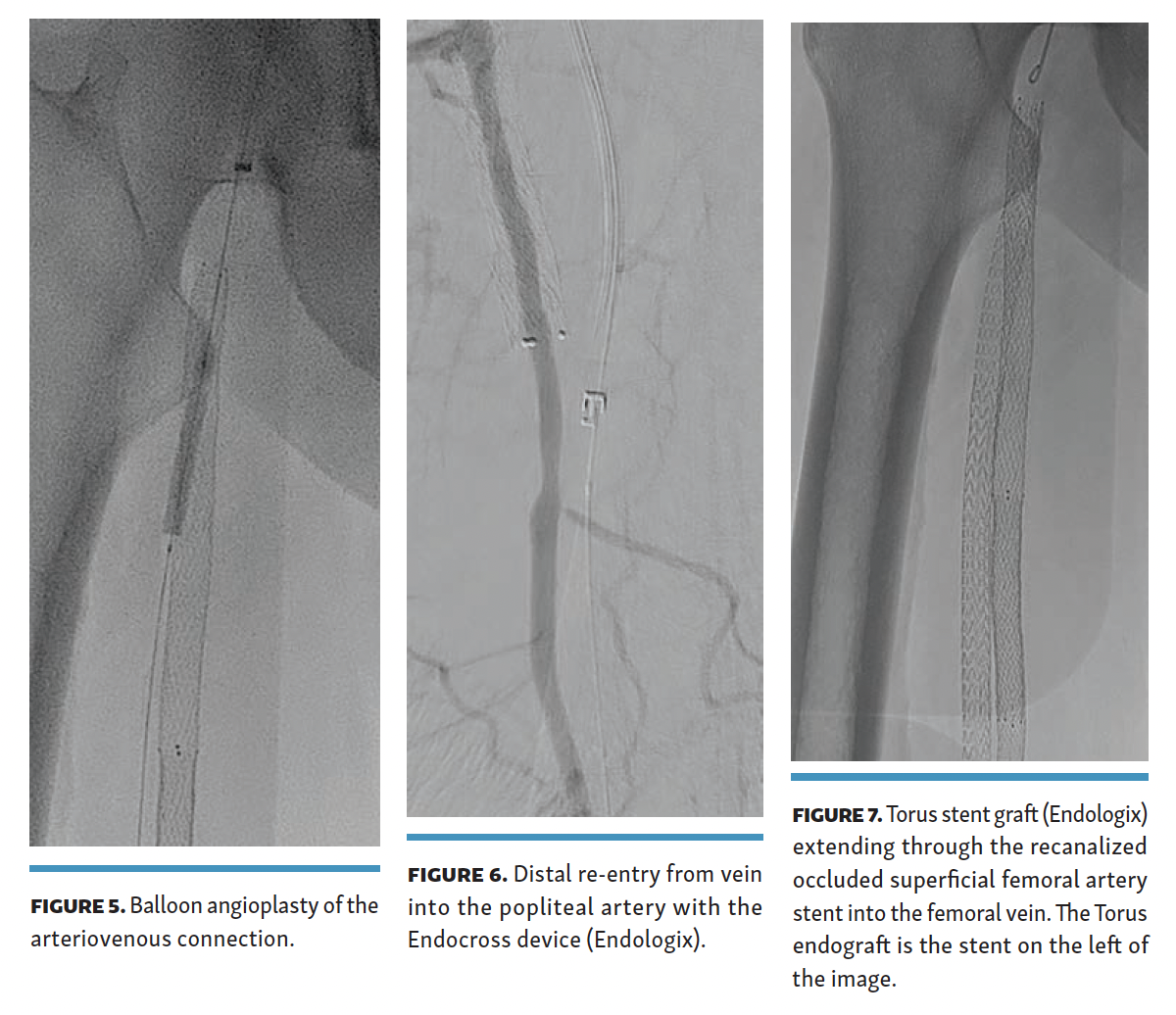
The arteriovenous passage was subsequently dilated with a 4-mm balloon (Figure 5). Distal re-entry was obtained in the above-knee popliteal artery (Figure 6), and 3 separate Torus covered stents were deployed from distal to proximal with a 6-cm overlap. No abrupt angulation was noted on fluoroscopy images between the prior occluded SFA stents and the thru-stent Torus endograft (Figure 7).
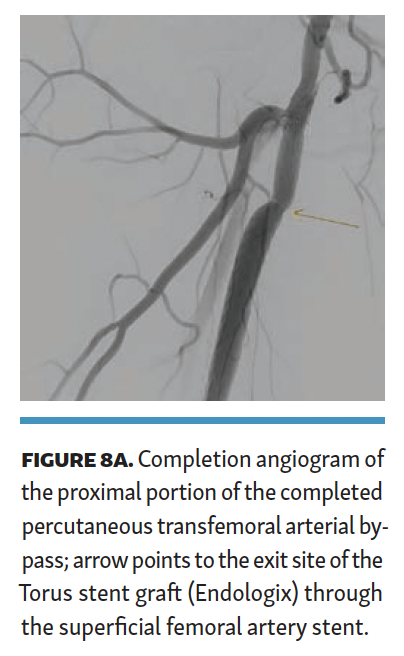
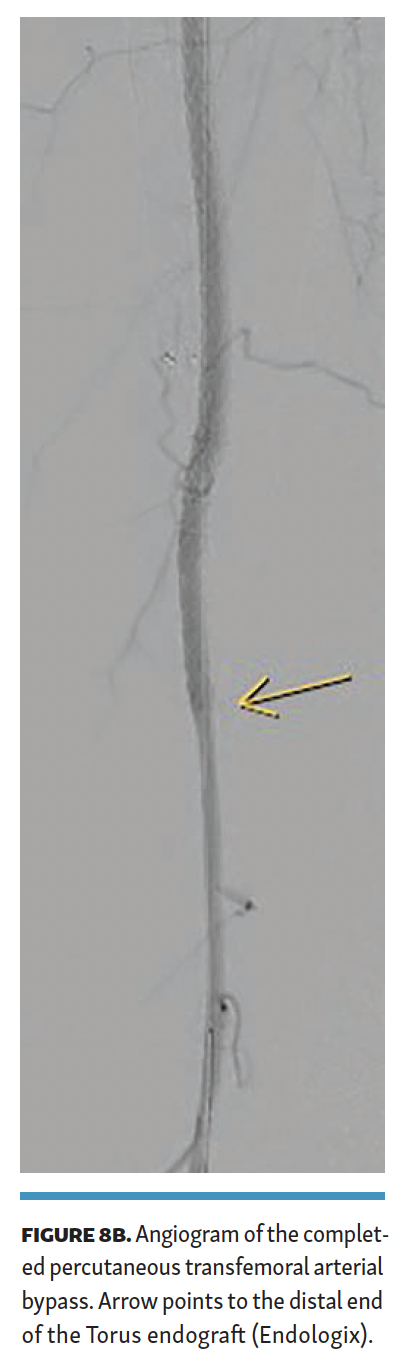 The Torus stent graft was then angioplastied with a high-pressure (24-ATM) balloon. Completion angiogram (Figures 8A and 8B) demonstrated brisk flow across the PTAB with no flow-limiting stenosis. The patient was discharged the next day. Total contrast volume was 40 mL; fluoroscopy time, 22.2 minutes; procedural time, 101 minutes; dose area product (DAP), 2874 µGym2; and air kerma (AK), 216 mGy. The patient was discharged on 20 mg of rivaroxaban and 81 mg of aspirin once a day.
The Torus stent graft was then angioplastied with a high-pressure (24-ATM) balloon. Completion angiogram (Figures 8A and 8B) demonstrated brisk flow across the PTAB with no flow-limiting stenosis. The patient was discharged the next day. Total contrast volume was 40 mL; fluoroscopy time, 22.2 minutes; procedural time, 101 minutes; dose area product (DAP), 2874 µGym2; and air kerma (AK), 216 mGy. The patient was discharged on 20 mg of rivaroxaban and 81 mg of aspirin once a day.
Follow-up and Outcomes
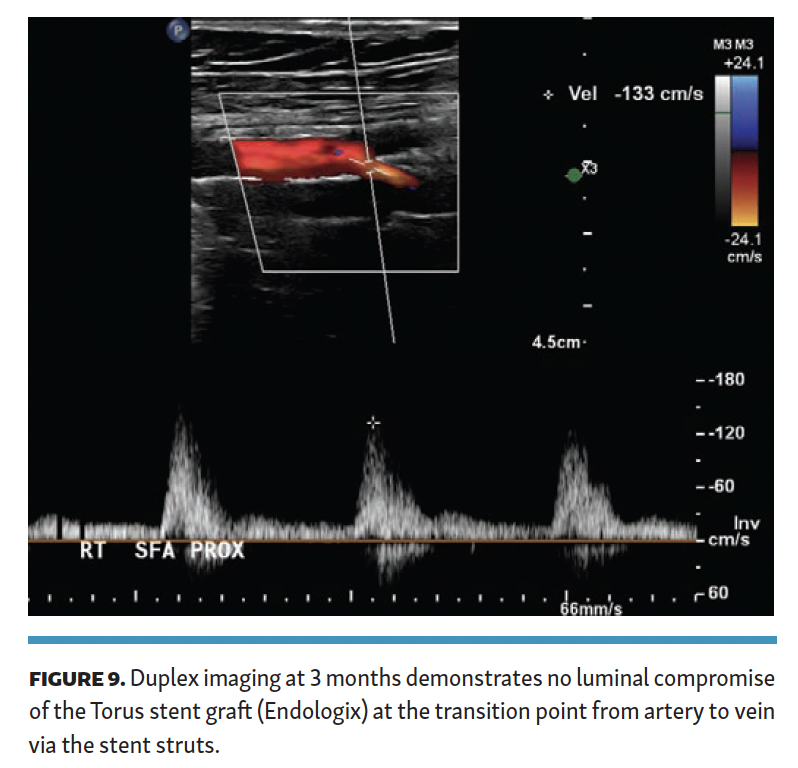
The patient was maintained on oral anticoagulation and aspirin. ABI at 6-month follow-up was 1.09, and she had no claudication symptoms. Duplex imaging of the Torus stent graft exiting the SFA across the stent into the femoral vein demonstrated no stenosis (Figure 9), with no velocity elevation at the proximal or distal end of the Torus stent. Duplex imaging did not demonstrate DVT in any of the veins in the target limb.
Case Report (Retro-Stent Technique)
Initial Presentation
A 68-year-old woman was referred for rest pain and nonhealing ulcers in her left lower extremity. The patient had 2 prior SFA stents (EverFlex) that had occluded. She subsequently had a femoral to above-knee bypass with a saphenous vein that had since occluded. The bypass graft occlusion was managed with thrombolysis and stenting of the proximal and distal ends of the vein graft. The patient now presented with rest pain/ulcers, and a computed tomography angiography (CTA) demonstrated occlusion of the bypass graft and prior SFA stents.
Procedural Overview
Contralateral arterial access (8F) and ipsilateral posterior tibial venous access were obtained (6F, 25-cm sheath). The 8F arterial sheath was advanced past the stent in the bypass graft, as this was extending a few millimeters into the common femoral artery. A 0.014 Glidewire Advantage Track wire was then advanced between the occluded SFA stent and the native SFA with the help of a Kumpe catheter (AngioDynamics). The angle of the Kumpe catheter was used to direct the wire in a posteromedial location to stay adjacent to the femoral vein. The retro-stent access track was then angioplastied with a 4-mm balloon (Figure 10). The Endocross was then advanced into this channel, and the crossing needle was deployed to gain access into the femoral vein 5 cm below the proximal end of the occluded SFA stent. The wire (0.014 Glidewire Advantage Track) in the femoral vein was subsequently snared, and through-and-through wire access was established. The 25-cm hydrophilic coated flexible tip of the 0.014 Glidewire Advantage Track with a stainless-steel core and polytetrafluoroethylene-coated body allows for easy maneuverability and the malleable tip allowing for flexibility during snare retrieval.
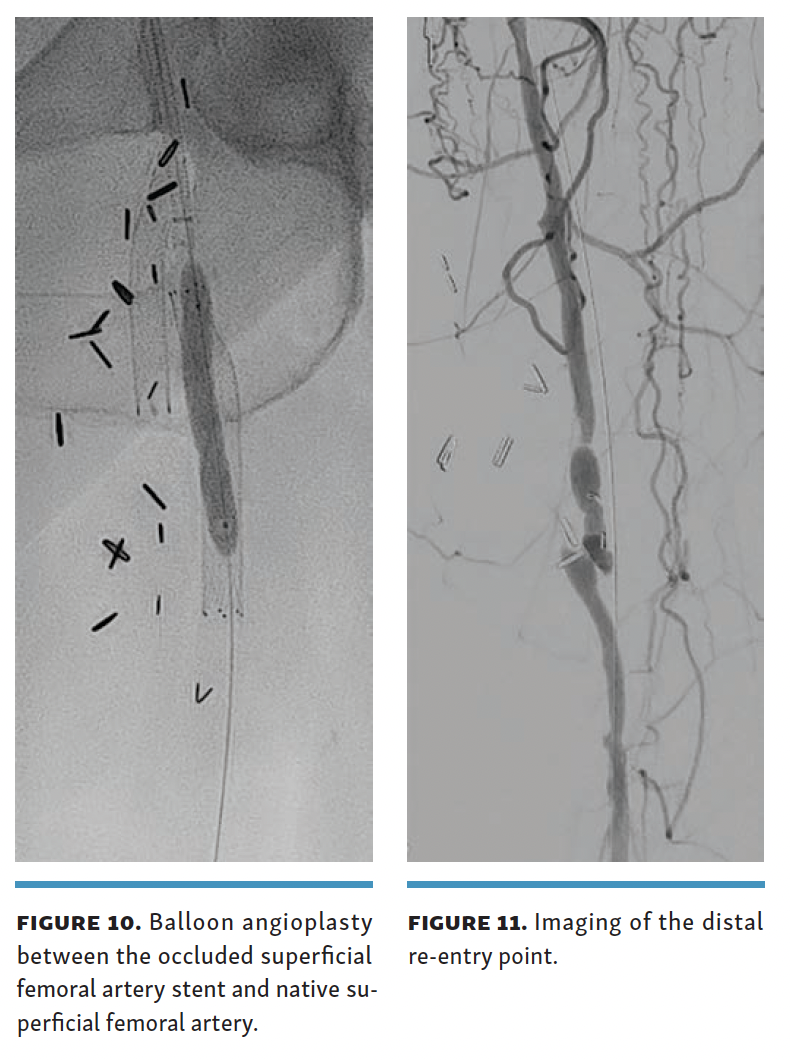
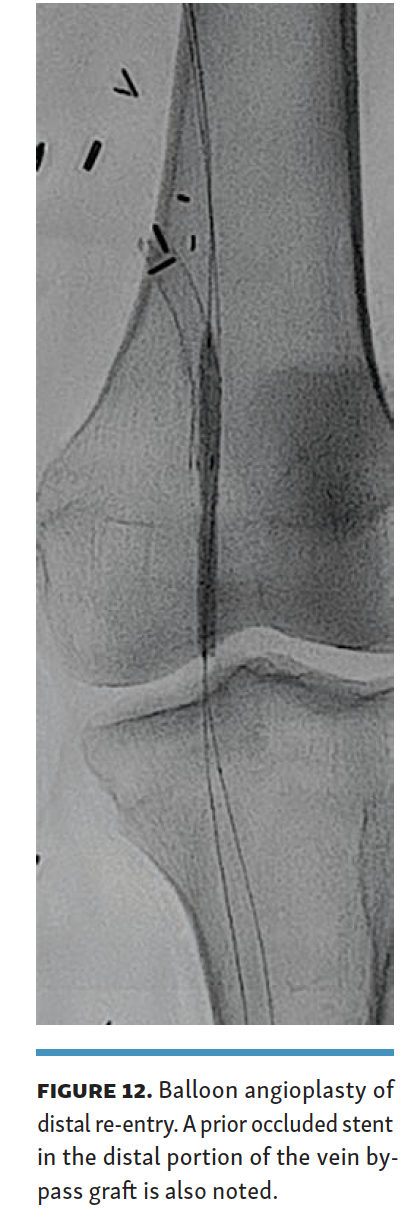
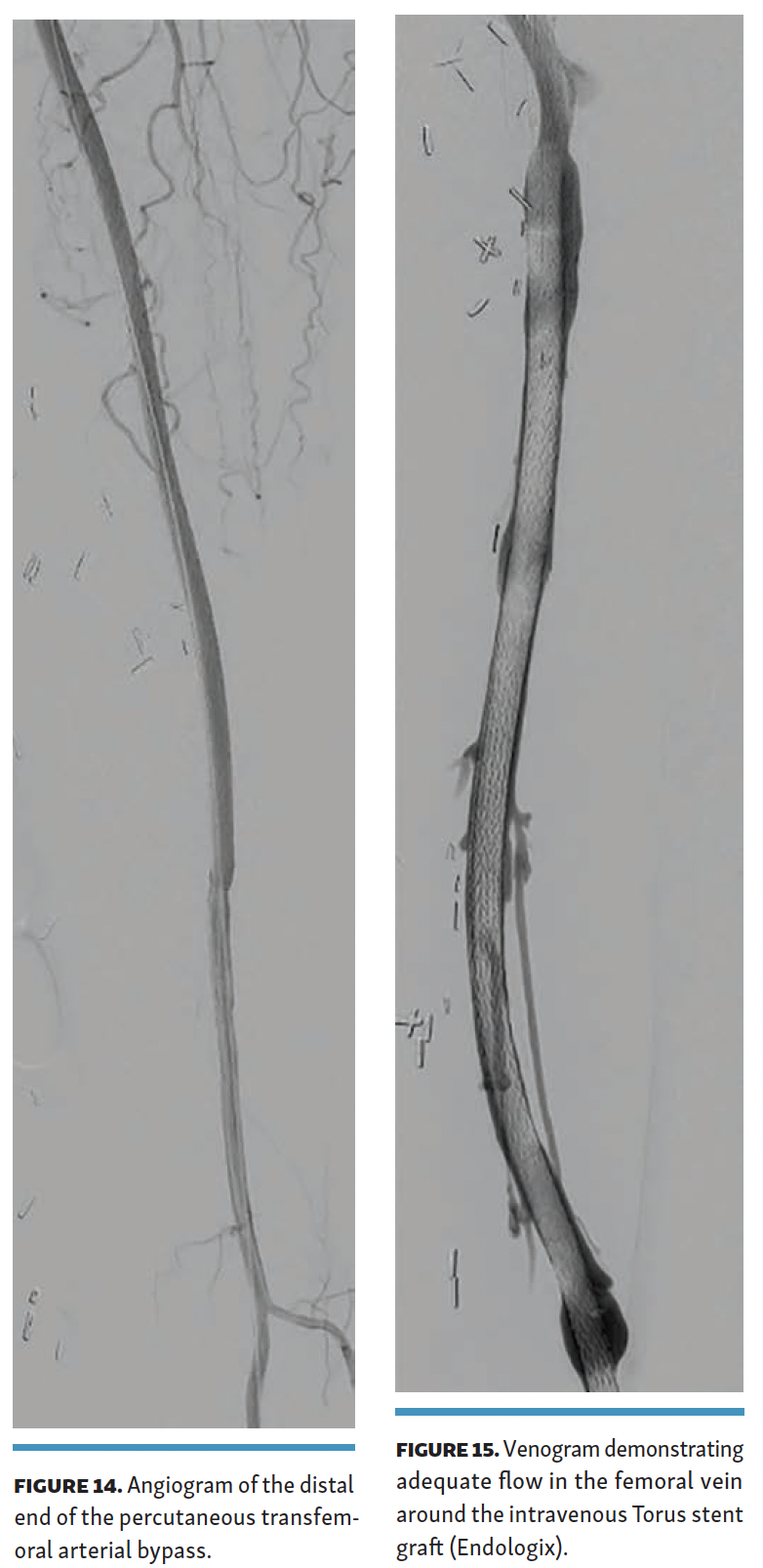
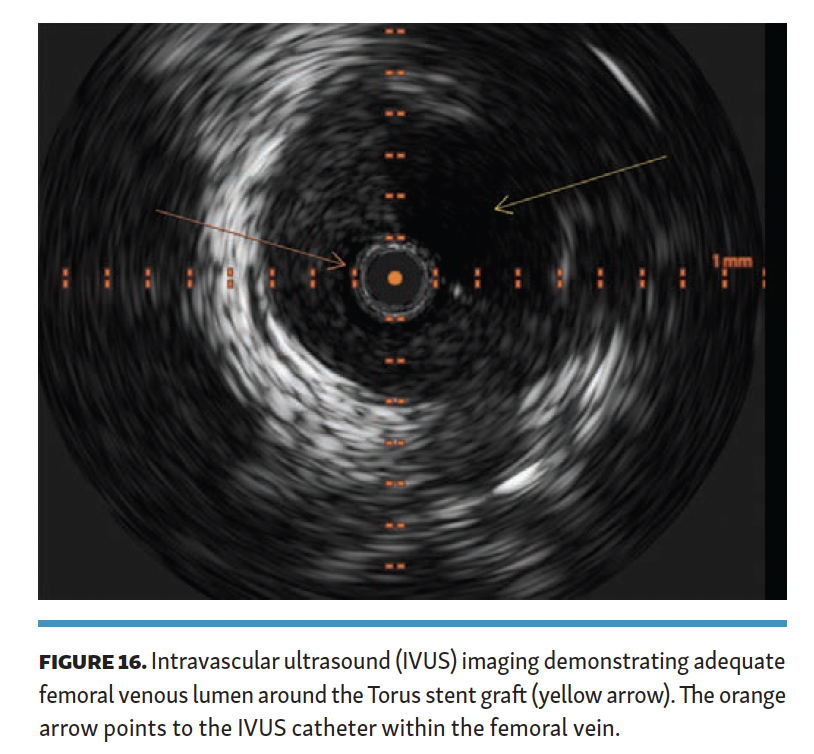
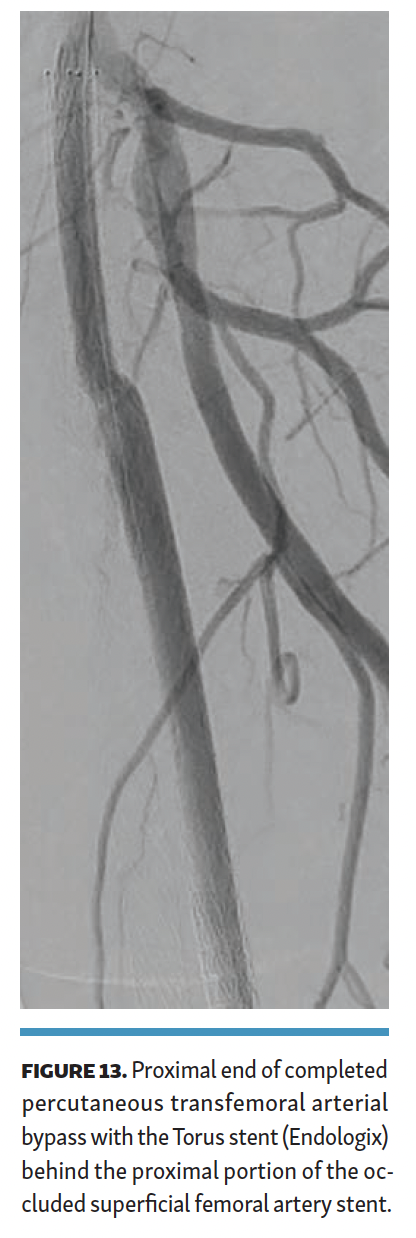 The arteriovenous connection was then ballooned, and the Endocross device was advanced into the femoral vein. Distal angiogram demonstrated multiple areas of occlusion in the distal SFA with the stent in the occluded vein bypass graft entering the proximal popliteal artery (Figure 11). Re-entry into the popliteal artery was obtained below this segment. Following dilation of the retry site (Figure 12), the Torus stent grafts were deployed distal to proximal. Completion angiogram after angioplasty of the stent graft demonstrated brisk flow proximally and into the popliteal artery (Figures 13 and 14). Venogram demonstrated unhindered flow through the femoral vein (Figure 15), and intravascular ultrasound imaging of the femoral vein demonstrated an adequate venous lumen next to the intravenous Torus stent graft (Figure 16). The patient was discharged the following day on 20 mg of rivaroxaban and 81 mg of aspirin once a day. Fluoroscopy of the proximal end demonstrated the Torus stent behind the prior SFA stent and the occluded stent in the proximal portion of the vein bypass graft (Figure 17). Procedural time was 139 minutes, fluoroscopy time 39 minutes, total contrast volume 100 mL, AK 332 mGy, and DAP 5550 µGym2.
The arteriovenous connection was then ballooned, and the Endocross device was advanced into the femoral vein. Distal angiogram demonstrated multiple areas of occlusion in the distal SFA with the stent in the occluded vein bypass graft entering the proximal popliteal artery (Figure 11). Re-entry into the popliteal artery was obtained below this segment. Following dilation of the retry site (Figure 12), the Torus stent grafts were deployed distal to proximal. Completion angiogram after angioplasty of the stent graft demonstrated brisk flow proximally and into the popliteal artery (Figures 13 and 14). Venogram demonstrated unhindered flow through the femoral vein (Figure 15), and intravascular ultrasound imaging of the femoral vein demonstrated an adequate venous lumen next to the intravenous Torus stent graft (Figure 16). The patient was discharged the following day on 20 mg of rivaroxaban and 81 mg of aspirin once a day. Fluoroscopy of the proximal end demonstrated the Torus stent behind the prior SFA stent and the occluded stent in the proximal portion of the vein bypass graft (Figure 17). Procedural time was 139 minutes, fluoroscopy time 39 minutes, total contrast volume 100 mL, AK 332 mGy, and DAP 5550 µGym2.
 Follow-up and Outcomes
Follow-up and Outcomes
The patient experienced mild reperfusion edema for approximately 1 month. Rest pain and ulcers had resolved by the 1-month visit. At the 2-month visit, the ABI was 1.12 with continued graft patency. Duplex imaging did not demonstrate DVT in any of the vessels in the target limb. Duplex imaging of the arterial system in the target limb demonstrated no significant velocity elevation or distal embolization.
Discussion
These case reports represent an innovative hybrid approach that physicians may consider in place of repeat endovascular recanalization or open surgical bypass. Despite early successful endovascular reinterventions for long-segment SFA lesions initially treated by a stent, the 1-year primary patency in early studies was as low as 46%.10 In 2010, Davies et al demonstrated comparable patency at 1 year with endovascular reinterventions vs open bypass (65% vs 67%, respectively) but at the cost of a significantly higher morbidity in the open group (16% vs 28%, respectively).11
Over time, with the evolution of techniques and technology, patency and outcomes have improved. Recent ACC/AHA/AAVPR/APMA/ABC/SCAI/SVM/SVN/SVS/SIR/VES guidelines (2024) support the use of endovascular therapies in the SFA, with 1A evidence for the use of endovascular revascularization to improve walking performance and quality of life in patients with inadequate response to noninvasive therapies, including medication and structured exercise.12 In addition to guideline support, a recent meta-analysis examined the results of 57 femoropopliteal studies and demonstrated that endovascular interventions, including atherectomy, bare metal stents (BMS) and drug-eluting stents (DES), covered stent grafts, drug-coated balloons (DCB), and intravascular lithotripsy, offer improved patency over percutaneous transluminal angioplasty (PTA) alone.13 In a number of studies using DCB, BMS, and DES in femoropopliteal lesions of less than 12 cm, 1-year primary patency was more than 70%.14-18 Given the variety of endovascular treatment options now available, a recent global algorithm for endovascular treatment has been proposed to help guide operators in the selection of endovascular approaches in the SFA.19 While endovascular treatment has proven more successful for shorter lesions, long femoropopliteal lesions can be technically challenging to cross and are more likely to fail due to restenosis. Historically, bypasses have been considered first-line therapy in SFA lesions of more than 25 cm, except in patients unfit for surgery.20
PTAB presents a new treatment option for patients with long femoropopliteal disease and is a less invasive option than open surgery while still providing comparable revascularization (Table). To our knowledge, the use of PTAB in patients with flush SFA stent occlusions has not been previously described based on an extensive literature search. In the DETOUR2 trial, 17.3% of patients had in-stent restenosis; however, none of these were stents extending into the proximal landing zone.9 Additionally, patients with prior open surgical bypass grafts were excluded from the DETOUR2 trial.9 However, as PTAB is approved for long-segment SFA occlusions both primary or recurrent, hence our use of the Detour system in both our study patients would be considered on-label. Both patients included in this case report had occluded stents extending into the proximal landing zone, requiring recrossing of the occluded SFA stents. One of the patients also had a prior open surgical bypass that had failed prior to the PTAB revascularization procedure. Despite these anatomic challenges, the PTAB device functioned seamlessly with continued patency and resolution of symptoms at the last follow-up.
Two different techniques were used to cross the occluded stents. The technique used was based on the ease of wire crossing, either through or behind the occluded stent. In neither technique, completion angiogram nor follow-up duplex imaging, demonstrated any flow compromise; however, the retro-technique had less proximal angulation of the torus stent graft on the completion angiogram (Figures 8A and 13). The retro-stent technique is more challenging due to the need to create a channel directed to the location of the femoral vein (posteromedial in most patients). If the channel created is located anterior or lateral to the occluded SFA stent, the Endocross would essentially have to cross 2 layers of nitinol stents to reach the femoral vein, which would create a greater risk of technical failure. Additionally, the Torus endograft would then have to traverse 2 layers of nitinol stents with increased risk for compression and stent fracture.
The presence of visible calcium on fluoroscopy can also make the retro-stent technique less attractive. Angioplasty of the channel between an occluded SFA stent and a noncompliant calcified vessel would increase the likelihood of vessel rupture and also limit the ability of the Torus endograft to fulling expand. Hence, if visible calcification is noted on fluoroscopy or pre-planning CTA, the thru-stent technique would be the preferred option.
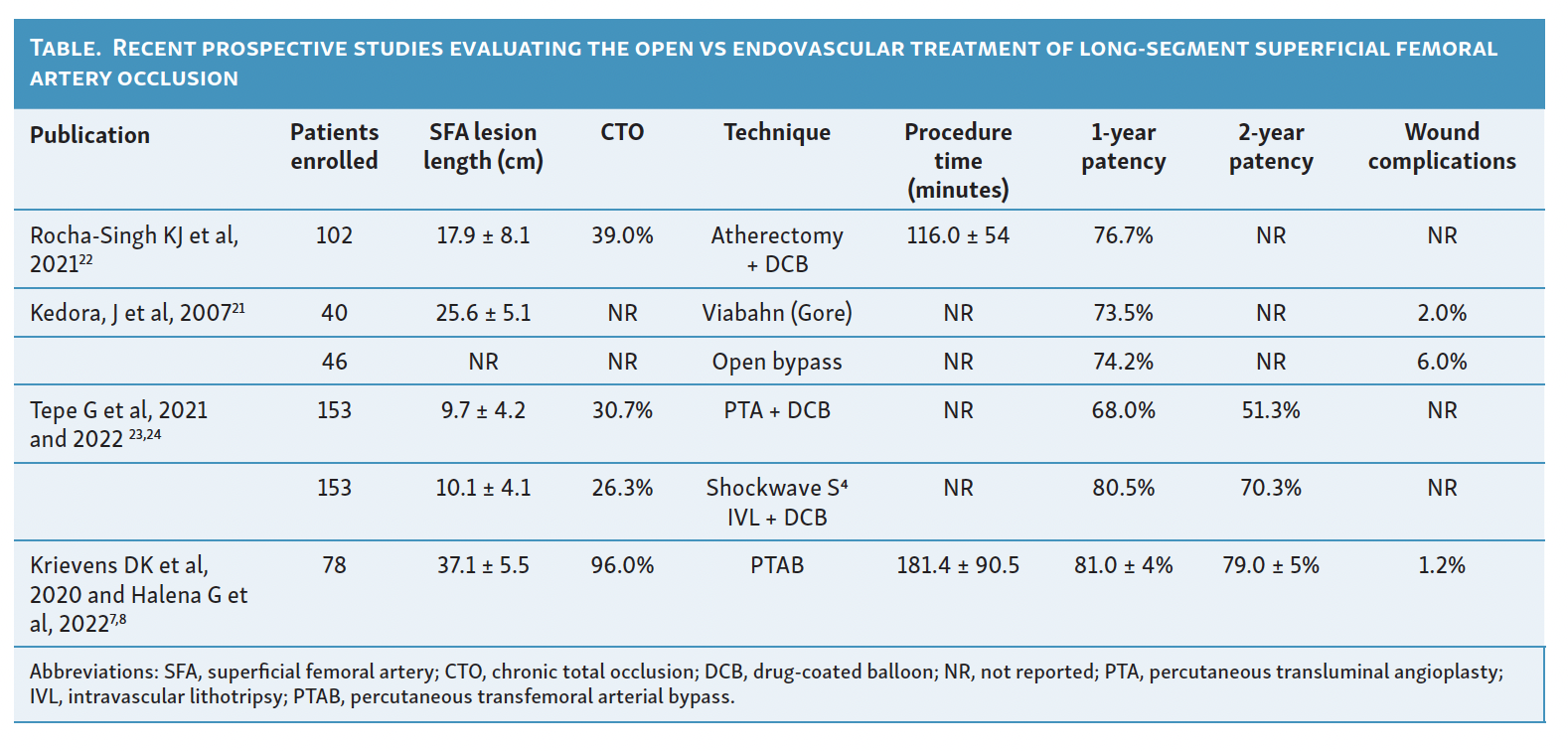
More recently, a meta-analysis of published studies (up to June 2019) for the endovascular treatment of long SFA lesions (mean lesion length of 269 mm) found that the primary patency rate at 1 and 2 years post-treatment was 62% and 55%, respectively.6 Variable endovascular techniques were used, making analysis difficult; overall, covered stents and drug-coated stents appear to have the most durable outcomes in this study.6 Recent prospective studies evaluating the open and endovascular treatment of long-segment SFA occlusions are reported in the Table. Endovascular treatments for long lesions, using a covered stent-graft system, had a 12-month primary patency of 73.5% with a reported mean lesion length of 264 mm.21 The REALITY study, a prospective single-arm trial that used a vessel preparation strategy with directional atherectomy prior to DCB angioplasty, included lesions that were a mean of 179 mm with a primary patency rate of 76.7% after 1 year.22 Data from the DETOUR2 trial, a prospective, single-arm, multicenter trial of the PTAB device for the treatment of long-segment SFA disease (mean lesion length 371 mm), showed a primary patency of 81.4% 12-months post-procedure.9 The PTAB trial data, despite enrolling patients with the longest lesion lengths (Table), had comparable or superior 1-year primary patency.7
Limitations
Our case study has a few limitations. This case report is limited to 2 patients and serves only as a technical note for performing PTAB in patients with flush SFA stent occlusions or prior failed bypass. The short follow-up interval on both patients (3 and 6 months) limits our ability to promote this technique as a durable procedure until long-term data is available on larger patient subsets. The long-term behavior of the Torus endograft, particularly regarding potential for material fatigue due to friction between the occluded SFA stent and the Torus endograft, could also be a cause for concern and would need additional benchtop fatigue testing and clinical study. Additionally, the use of this technique in patients with different SFA stent configurations such as covered stents, Supera stent (Abbott Vascular), etc, needs further study. Both our patients had the EverFlex nitinol stents placed during the prior SFA intervention. Further analysis with a larger number of patients with flush SFA stent occlusions is needed to confirm and extend these findings.
Conclusions
This approach, restoring blood flow to the lower leg by constructing a bypass using the femoral vein to divert circulation around the obstructed SFA, could represent an important advancement in treating patients who develop late stent occlusions in the SFA, particularly when traditional recanalization or open surgery is not feasible.
Affiliations and Disclosures
From the Department of Surgery, Stanford Health Care TriValley, Pleasanton, California.
Acknowledgment: The author thanks Heather Gorby, PhD, under contract to Endologix, for medical writing support.
Disclosure: Dr Kasirajan is a paid consultant to Endologix.
Manuscript accepted November 10, 2025.
Corresponding Author: Karthikeshwar Kasirajan, MD, Stanford Health Care TriValley, 5565 W. Las Positas Blvd, Pleasanton, CA 94588. Email: kkasirajan@stanford.edu
References
1. Beckman JA, Schneider PA, Conte MS. Advances in revascularization for peripheral artery disease: revascularization in PAD. Circ Res. 2021;128(12):1885-1912. doi:10.1161/CIRCRESAHA.121.318261
2. AbuRahma AF. When are endovascular and open bypass treatments preferred for femoropopliteal occlusive disease? Ann Vasc Dis. 2018;11(1):2 doi:10.3400/avd.ra.18-00001.5-40
3. Bailey SR, Beckman JA, Dao TD, et al. ACC/AHA/SCAI/SIR/SVM 2018 Appropriate Use Criteria for Peripheral Artery Intervention: a report of the American College of Cardiology Appropriate Use Criteria Task Force, American Heart Association, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, and Society for Vascular Medicine. J Am Coll Cardiol. 2019;73(2):214-237. doi:10.1016/j.jacc.2018.10.002
4. Litsky J, Chanda A, Stilp E, Lansky A, Mena C. Critical evaluation of stents in the peripheral arterial disease of the superficial femoral artery - focus on the paclitaxel eluting stent. Med Devices (Auckl). 2014;7:149-156. doi:10.2147/MDER.S45472
5. Lensvelt MMA, Reijnen MMPJ, Wallis De Vries BM, Zeebregts CJ. Treatment strategies for extensive chronic SFA occlusions: indications and results. J Cardiovasc Surg (Torino). 2012;53(1 Suppl 1):161-170.
6. Giannopoulos S, Lyden SP, Bisdas T, et al. Endovascular intervention for the treatment of Trans-Atlantic Inter-Society Consensus (TASC) D femoropopliteal lesions: a systematic review and meta-analysis. Cardiovasc Revasc Med. 2021;22:52-65. doi:10.1016/j.carrev.2020.06.014
7. Krievins DK, Halena G, Scheinert D, et al. One-year results from the DETOUR I trial of the PQ Bypass DETOUR System for percutaneous femoropopliteal bypass. J Vasc Surg. 2020;72(5):1648-1658.e2. doi:10.1016/j.jvs.2020.02.043
8. Halena G, Krievins DK, Scheinert D, et al. Percutaneous femoropopliteal bypass: 2-year results of the DETOUR system. J Endovasc Ther. 2022;29(1):84-95. doi:10.1177/15266028211034862
9. Lyden SP, Soukas PA, De A, et al. DETOUR2 trial outcomes demonstrate clinical utility of percutaneous transmural bypass for the treatment of long segment, complex femoropopliteal disease. J Vasc Surg. 2024;79(6):1420-1427.e2. doi:10.1016/j.jvs.2024.02.004
10. Gray BH, Sullivan TM, Childs MB, Young JR, Olin JW. High incidence of restenosis/reocclusion of stents in the percutaneous treatment of long-segment superficial femoral artery disease after suboptimal angioplasty. J Vasc Surg. 1997;25(1):74-83.
11. Davies MG, Bismuth J, Saad WE, Naoum JJ, Peden EK, Lumsden AB. Outcomes of interventions for recurrent disease after endoluminal intervention for superficial femoral artery disease. J Vasc Surg. 2010;52(2):331-339.e1-2. doi:10.1016/j.jvs.2010.02.278
12. Gornik HL, Aronow HD, Goodney PP, et al; Peer Review Committee Members. 2024 ACC/AHA/AACVPR/APMA/ABC/SCAI/SVM/SVN/SVS/SIR/VESS Guideline for the management of lower extremity peripheral artery disease: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2024;149(24):e1313-e1410. doi:10.1161/CIR.0000000000001251
13. Schwartz AW, Shah Y, Huang H, et al. Comparison of endovascular interventions for the treatment of superficial femoral artery disease: a network meta-analysis. J Soc Cardiovasc Angiogr Interv. 2025:4(1):102432. doi:10.1016/j.jscai.2024.102432
14. Dake MD, Ansel GM, Jaff MR, et al; Zilver PTX Investigators. Paclitaxel-eluting stents show superiority to balloon angioplasty and bare metal stents in femoropopliteal disease: twelve-month Zilver PTX randomized study results. Circ Cardiovasc Interv. 2011;4(5):495-504. doi:10.1161/CIRCINTERVENTIONS.111.962324
15. Garcia L, Jaff MR, Metzger C, et al; SUPERB Trial Investigators. Wire-interwoven nitinol stent outcome in the superficial femoral and proximal popliteal arteries: twelve-month results of the SUPERB trial. Circ Cardiovasc Interv. 2015;8(5):e000937. doi:10.1161/CIRCINTERVENTIONS.113.000937
16. Laird JR, Katzen BT, Scheinert D, et al; RESILIENT Investigators. Nitinol stent implantation versus balloon angioplasty for lesions in the superficial femoral artery and proximal popliteal artery: twelve-month results from the RESILIENT randomized trial. Circ Cardiovasc Interv. 2010;3(3):267-276. doi:10.1161/CIRCINTERVENTIONS.109.903468
17. Tepe G, Laird J, Schneider P, et al; IN.PACT SFA Trial Investigators. Drug-coated balloon versus standard percutaneous transluminal angioplasty for the treatment of superficial femoral and popliteal peripheral artery disease: 12-month results from the IN.PACT SFA randomized trial. Circulation. 2015;131(5):495-502. doi:10.1161/CIRCULATIONAHA.114.011004
18. Zeller T, Tiefenbacher C, Steinkamp HJ, et al. Nitinol stent implantation in TASC A and B superficial femoral artery lesions: the Femoral Artery Conformexx Trial (FACT). J Endovasc Ther. 2008;15(4):390-398. doi:10.1583/08-2461.1
19. Korosoglou G, Schmidt A, Lichtenberg M, et al. Global algorithm for the endovascular treatment of chronic femoropopliteal lesions: an interdisciplinary expert opinion statement. J Am Coll Cardiovasc Interv. 2025;18(5):545–557. doi:10.1016/j.jcin.2024.11.038
20. Aboyans V, Ricco J, Bartelink MEL, et al. Editor’s Choice – 2017 ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in collaboration with the European Society for Vascular Surgery (ESVS). Eur J Vasc Endovasc Surg. 2018;55(3):305-368. doi:10.1016/j.ejvs.2017.07.018
21. Kedora J, Hohmann S, Garrett W, Munschaur C, Theune B, Gable D. Randomized comparison of percutaneous Viabahn stent grafts vs prosthetic femoral-popliteal bypass in the treatment of superficial femoral arterial occlusive disease. J Vasc Surg. 2007;45(1):10-16. doi:10.1016/j.jvs.2006.08.074
22. Rocha-Singh KJ, Sachar R, DeRubertis BG, et al; REALITY Investigators. Directional atherectomy before paclitaxel coated balloon angioplasty in complex femoropopliteal disease: The VIVA REALITY study. Catheter Cardiovasc Interv. 2021;98(3):549-558. doi:10.1002/ccd.29777
23. Tepe G, Brodmann M, Werner M, et al; DISRUPT PAD III Investigators. Intravascular lithotripsy for peripheral artery calcification. JACC Cardiovasc Interv. 2021;14(12):1352-1361. doi:10.1016/j.jcin.2021.04.010
24. Tepe G, Brodmann M, Bachinsky W, et al. Intravascular lithotripsy for peripheral artery calcification: mid-term outcomes from the randomized Disrupt PAD III trial. J Soc Cardiovasc Angiogr Interv. 2022;1(4):100341. doi:10.1016/j.jscai.2022.100341















