A Tale of Two Agencies: Medical Device Approval and Coverage in the United States
© 2025 HMP Global. All Rights Reserved.
Any views and opinions expressed are those of the author(s) and/or participants and do not necessarily reflect the views, policy, or position of Cath Lab Digest or HMP Global, their employees, and affiliates.
Mehdi H. Shishehbor, DO, MPH, PhD1,2 and Ashkan Yahyavi, MD1
1University Hospitals Harrington Heart & Vascular Institute; 2Case Western Reserve University School of Medicine, Cleveland, Ohio
Disclosures: MHS: Advisory and consultant for Abbott Vascular, Medtronic, Terumo, Boston Scientific, Philips, ANT, Inquis Medical, and Inari Medical. AY: None.
The authors can be contacted via Mehdi H. Shishehbor, DO, MPH, PhD, at mehdi.shishehbor@uhhospitals.org
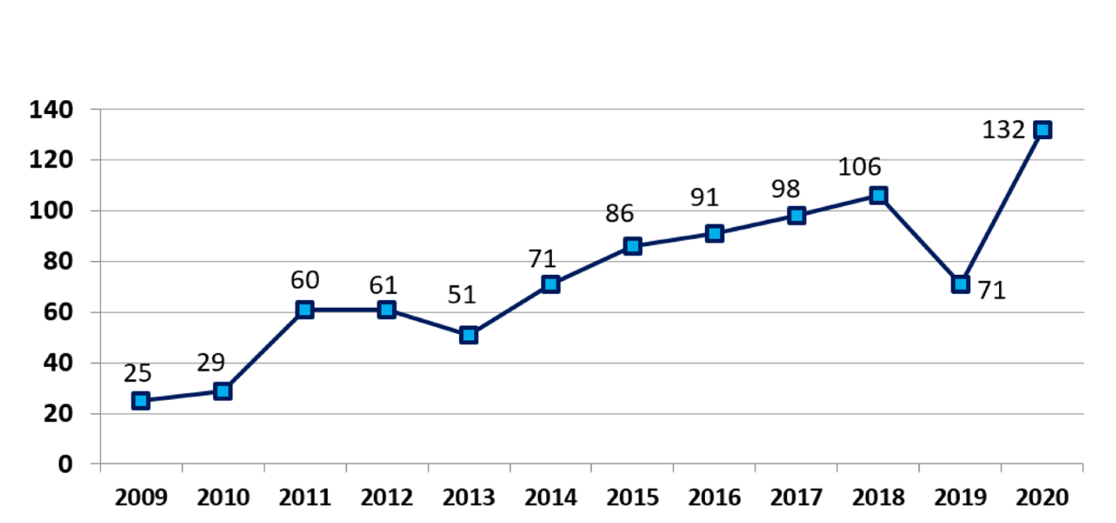
Health coverage in the United States is a challenging environment. While much of the focus has been on denial and delay in healthcare coverage, the critical issue of approving and covering new medical devices and technologies has not been adequately addressed. Every year, the FDA approves numerous innovative medical devices that have the potential to impact patient care and improve health outcomes. However, without the CMS approval for coverage, these innovations rarely reach the patients in a timely fashion. In recent years, we have observed increasing FDA approvals of new technologies (Figure); but the CMS coverage has lagged.
The FDA, as the leading federal agency dedicated to ensuring the safety and efficacy of medical products, including new devices and technologies, implements a rigorous approval process that demands significant time and resources. This process begins with a thorough review of preliminary data from in-vitro, animal, or human studies. Depending on the risk classification, devices undergo various phases of meticulous review by FDA experts to confirm their safety and effectiveness. This includes an exhaustive examination of clinical trial data, manufacturing information, and proposed labeling. Importantly, the safety monitoring process does not end with approval; the FDA continues to collect data on any safety or effectiveness concerns even after a medical device is approved for marketing in the US. The process to assure safety and efficacy is completely independent of coverage which typically follows FDA approval.
According to statistics, the number of FDA approvals for new medical devices has steadily increased over the past 15 years, reaching a notable count of 132 in 2020 (Figure). This trend is projected to continue, driven by the ongoing development of innovative devices and technologies.1
Once FDA approval has been granted, the CMS and third party payers (health insurance companies) determine if medical devices are eligible for coverage. Obtaining Medicare coverage through a National Coverage Determination (NCD) by CMS is a thorough and evidence-based process. It begins when CMS formally receives an NCD request from either an external stakeholder or internally from CMS staff. Upon acceptance, CMS posts a tracking sheet on their coverage website, informing the public that an item or service is under review. This National Coverage Analysis (NCA) process involves a meticulous examination of scientific data, including clinical trial results, to determine whether the device meets Medicare coverage requirements. The public can participate and comment during this process. Ideally, the NCA process should take nine to twelve months but recent study has shown it can take a median of 5.7 years.2 If the device meets the necessary criteria, CMS issues an NCD, confirming nationwide coverage under Medicare.3 Additionally, the Medicare program can grant coverage to new medical devices through local coverage by Medicare administrative contractors or through claim-by-claim adjudication when formal national or local coverage policies are not developed.4
Despite previous efforts by CMS to reduce the gap between regulatory approval and Medicare coverage, such as the introduction of the Transitional Coverage for Emerging Technologies (TCET) pathway in August 2024,5 the rate of CMS coverage approvals will likely continue to lag behind that of the FDA. This discrepancy is primarily due to insufficient funding and administrative resources. Historically, inadequate staffing and funding, stemming from the extensive bureaucracy involved in the CMS approval process, have hindered the timely evaluation and coverage of innovative medical devices, ultimately delaying patient access to critical healthcare advancements.2,6
Notably, even among the devices that receive NCDs, coverage is often conditional and frequently requires additional data collection if CMS determines that the device does not fully meet its somewhat vaguely defined "reasonable and necessary" standard for reimbursement. Furthermore, data from previous studies suggest that private payers disagree with CMS coverage decisions nearly half the time.7
This stringent approach, coupled with the large number of new devices awaiting the lengthy administrative process for potential coverage approval, has caused dissatisfaction among many healthcare beneficiaries in recent years. Despite CMS's efforts to codify the definition of "reasonable and necessary," the resulting inconsistent coverage across different geographic regions for many new devices and technologies further exacerbates this dissatisfaction.
The issues outlined above have significant implications for patients. The restricted availability of new devices and technologies due to lack of coverage is creating a notable disparity in access to advanced medical care. Physicians frequently encounter situations where patients refuse newer, more efficient treatments involving medical devices or technologies because of insufficient healthcare coverage. In the long run, this can potentially have an adverse impact on patient health outcomes across various medical disciplines and clinical settings.
The conundrum extends beyond patients. Hospitals also face significant financial challenges when providing medical services without reimbursement. Without the financial support, hospitals and physicians are often unable to adopt new, potentially lifesaving technologies in a timely manner.8,9 This effect is possibly more pronounced in smaller hospitals and those serving underprivileged communities.
Resolving this ever-growing healthcare issue requires policymakers to work towards closing the gap between FDA approval and CMS coverage. Currently, several programs aim to improve the speed and effectiveness of the CMS approval process, such as the Parallel Review Program, Payor Communication Task Force, and Early Payor Feedback Program. These initiatives are notable efforts to enhance communication between the FDA and CMS and address the problem. However, the current state of the issue underscores the need for much greater alignment between the two agencies and the importance of a collaborative effort to develop standard nationwide protocols for decision-making. Ideally, this could result in substantial long-term improvements in patient outcomes and enable the healthcare system to deliver high-quality care while more easily navigating financial challenges. Lastly, a commitment from third party payers to follow CMS coverage decisions would significantly improve quality of care and accessibility of new technologies and medications.
References
-
Shuren J. Reflections on a Record Year for Novel Device Innovation Despite COVID-19 Challenges 2021 [October 2024]. Available from: https://www.fda.gov/news-events/fda-voices/reflections-record-year-novel-device-innovation-despite-covid-19-challenges.
-
Sexton ZA, Perl JR, Saul HR, Trotsyuk AA, Pietzsch JB, Ruggles SW, et al, eds. Time from authorization by the US Food and Drug Administration to Medicare coverage for novel technologies. JAMA Health Forum. 2023 Aug 4;4(8):e232260. doi:10.1001/jamahealthforum.2023.2260
-
(CMS) CfMMS. Guide for Medical Technology Companies and Other Interested Parties 2024 [October 2024]. Available from: https://www.cms.gov/medicare/coding-billing/guide-medical-technology-companies-other-interested-parties.
-
Centers for Medicare & Medicaid Services. Local Coverage Determination Process and Timeline [Internet]. Available from: https://www.cms.gov/cms-guide-medical-technology-companies-and-other-interested-parties/coverage/local-coverage-determination-process-and-timeline.
-
Services CfMM. Final Notice — Transitional Coverage for Emerging Technologies (CMS-3421-FN) 2024 [October 2024]. Available from: https://www.cms.gov/newsroom/fact-sheets/final-notice-transitional-coverage-emerging-technologies-cms-3421-fn.
-
Zinn AM, Allen Jr JC, Hacker CS. Median approval times for class III medical devices have been well above statutory deadlines set for FDA and CMS. Health Aff (Millwood). 2012 Oct;31(10):2304-13. doi:10.1377/hlthaff.2010.1198
-
Chambers JD, Chenoweth M, Thorat T, Neumann PJ. Private payers disagree with Medicare over medical device coverage about half the time. Health Aff (Millwood). 2015 Aug;34(8):1376-1382. doi:10.1377/hlthaff.2015.0133.
-
Shishehbor MH, Jaff MR, Beckman JA, Misra S, Schneider PA, Lookstein R, et al. Public health impact of the centers for medicare and medicaid services decision on pass-through add-on payments for drug-coated balloons: A call to action. JACC Cardiovasc Interv. 2018 Mar 12;11(5):496-499. doi:10.1016/j.jcin.2018.01.233.
-
Shishehbor MH, White CJ, Beckman JA, et al. Centers for Medicare & Medicaid Services’ decision on drug-coated balloons: No additional reimbursement despite higher cost and highest levels of scientific evidence. Vasc Med. 2018 Dec;23(6):558-559. doi:10.1177/1358863X18802969










