The Elusive Definitive Device for Amputation Reduction
© 2025 HMP Global. All Rights Reserved.
Any views and opinions expressed are those of the author(s) and/or participants and do not necessarily reflect the views, policy, or position of Cath Lab Digest or HMP Global, their employees, and affiliates.
Mark C. Bates, MD, DSc (Hon), FACC, FSCAI,
is currently an Innovation Champion for W.L. Gore & Associates and Board Chair for Avobis Bio. He is an Emeritus Senior Scientist at the CAMC Institute of Academic Medicine and Clinical Professor, Medicine and Surgery at West Virginia University School of Medicine. He also is the founder of the CAMC Vascular Center of Excellence in Charleston, West Virginia.
Learn more about Dr. Bates here.
Dr. Bates can be contacted at markcbatesmd@icloud.com
 How have amputation rates among lower-extremity peripheral artery disease (PAD) patients changed since the widespread adoption of endovascular interventions, particularly in patients with chronic limb-threatening ischemia (CLTI)?
How have amputation rates among lower-extremity peripheral artery disease (PAD) patients changed since the widespread adoption of endovascular interventions, particularly in patients with chronic limb-threatening ischemia (CLTI)?
Twenty years ago, we saw promising improvements in CLTI outcomes that seemed to parallel the advent of less invasive therapies. However, in 2021, the American Heart Association issued a policy statement in response to a 50% increase in non-traumatic amputations between 2009 and 2015, with particularly disturbing findings in the diabetic population.1 The policy statement was very well written, emphasizing early diagnosis and best medical therapy, since this is the mainstay of amputation prevention. While this increase in amputation rate has occurred despite a significant increase in interventional procedures, I am not suggesting there are adequate data to indicate a cause and effect. There are many variables in play, including the increasing incidence of diabetes, disparity of care, and the aging population. Regardless, it does seem we are losing the battle here, and what if we are missing something or making some patients worse?
Are there specific endovascular techniques or devices that have demonstrated significantly better limb salvage outcomes than others?
The short answer here is no. Most studies are observational, registry-based, or limited by selection bias and confounding variables. Even the recently celebrated LIFE-BTK trial2 admittedly included shorter lesions than generally encountered in clinical practice and required screening nearly 8000 patients to enroll 261 patients. More importantly, the trial relied on a composite endpoint that was weighted by vessel patency as a surrogate for clinical outcomes. While the composite endpoint underscores the scaffold’s enhanced vessel patency over angioplasty alone, it was disappointing to see 4 amputations in the treatment arm at 1 year versus none in the angioplasty control.2 Additionally, wound healing — a critical measure of clinical success in CLTI — was not a powered endpoint in the study and descriptive analyses indicated 1-year wound healing rates were essentially the same in both groups (scaffold group 76.1% versus PTA control of 80%).2 I am not suggesting the bioresorbable scaffold increased amputation since this was a 2:1 randomization, and chance likely explains that finding. It illustrates the challenge of actual clinical endpoints even when you select patients without significant distal disease. Remember, CLTI is often the end-stage manifestation of a systemic disease with heterogeneity of disease complexity, phenotypes, wound care, social determinants of health, and endovascular treatment strategies. Thus, adequately powered trials for meaningful clinical outcomes have been cost-prohibitive but continued reliance on clinical surrogates does leave some uncertainty in the context of this discussion. I believe the BTK bioresorbable scaffold is a step forward in patency and trial design, so it is a needed advance, but unlikely to be a definitive solution for all CLTI patients.
How concerned are you about the risk of embolic debris during endovascular interventions?
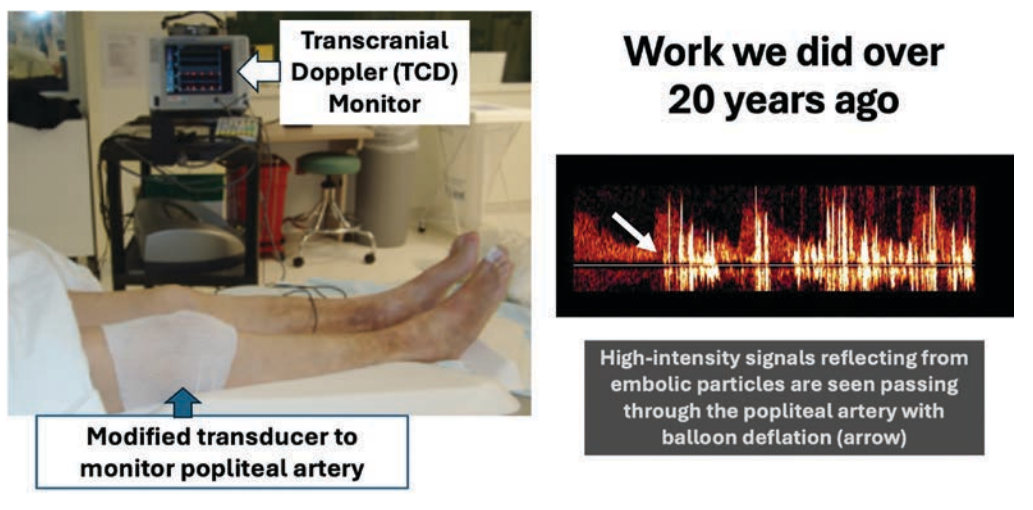
My concern about embolization during interventional procedures dates back almost 3 decades. While researching and developing one of the early carotid filter systems and as a co-inventor of carotid flow reversal, it became apparent how little we knew about particle release during all interventions. We subsequently did studies utilizing a modified transcranial Doppler system to do popliteal surveillance during various peripheral procedures and found embolic storms were common (Figure 1). Many studies have proven significant debris is captured in distal filters during peripheral intervention, with the highest embolic burden noted following atherectomy.3 The challenge has always been adjudicating whether this is clinically significant, and many have argued these distal vessels recanalize. However, what if the long-term loss for flow reserve increases later risk of CLTI? The landmark study in JACC by Narula et al4 on the systematic assessment of below-the-knee (BTK) arteries in amputated limbs showed the shocking finding that much of the distal occlusions were from chronic embolization due to the dynamic changes of plaque ulceration and thrombosis in the more proximal plaque. Over one-half of the occluded BTK vessels were the result of thrombus without significant disease. This explains why the only treatment, outside stopping smoking and disease modification drugs, for reducing amputation in claudication patients is a NOAC. More importantly, if this is a contributing factor for CLTI in nature, then how can it not also be the case from the iatrogenic release of thrombus, plaque fragments, cholesterol crystals, and other debris during multilevel intervention?

Can you discuss the relationship between embolic debris released during procedures and the potential for worsening PAD over time?
This is the hypothesis of the “inflow-outflow paradox”: is that gratifying angiographic finding after excavation of a long superficial femoral artery (SFA) occlusion or treatment of multilevel disease counterbalanced by an unseen chronic paradoxical reduction in outflow? There are some signals from the pharma trials and emerging clinical trial data suggesting claudication patients who undergo intervention may have higher long-term risk of amputation that increases with the number of repeat procedures, early treatment paradigms, and perhaps when atherectomy is used (Figure 2). When I started my practice 30 years ago, we would always educate claudication patients that no surgical or endovascular procedure has ever been shown to reduce the risk of amputation or make people live longer. I don’t think it is time to say intervention may increase amputation, but are we missing something that could be important to ensure that is not the case?
What steps do you take to mitigate the risk of embolic debris during interventions?
First, there is no empirical evidence that protection systems change outcomes and thus, I am cautious about sharing anecdotal experience since we need to move from gestalt to evidence-based decisions. With that preface, it’s very case specific. We tend to use distal protection systems for complex atherectomy procedures or patients with extremely poor runoff. If there is a high burden of soft plaque in a long SFA occlusion, I would often place a blood pressure cuff on the calf and inflate it above the blood pressure for brief periods during times in the procedure with the highest embolic load. This causes flow arrest in the outflow, and allows for a suction catheter to be placed in the distal popliteal artery to remove debris, before allowing distal flow. We have used balloon tip sheaths to dilate common iliac occlusions that are at high risk of embolization, as examples. However, there is no ideal solution for all patients, and it really comes down to patient selection.
Could you describe the significance of adequate outflow tract in determining procedural success in lower-extremity revascularization?
One of the biggest variables early on in femoral popliteal bypass graft patency was distal runoff and this holds true for endovascular procedures as well. Of course, establishing in-line flow to the tissue target angiosome is critical for wound healing.
In your experience, how does the condition of the outflow tract correlate with overall limb salvage rates and long-term patency?
As I noted, many CLTI BTK device trials now require no significant disease from 10 centimeters above the ankle down into the foot. There has been tribal knowledge for many years that the patients who do best with BTK intervention are the non-diabetics with isolated proximal tibioperoneal disease. However, this made up less than a quarter of cases in my practice. AI-enabled data now provides increased clarity on angiographic phenotypes associated with worse outcomes. It is no surprise that distal disease is an important variable. We all see this distal disease issue as in itself causative, but it is also possible that the type of patients that have this type of disease are those with severe end-organ and systemic disease.
How can we better identify which patients are most at risk for clinically significant embolization, and are there AI tools emerging that can help stratify these risks across different lesion types or patient subsets?
What’s still missing, at least for me, is our ability to account for the heterogeneity in patient phenotype when looking at current AI models. There are efforts underway. Stanford and Mayo Clinic, among others, are exploring this, but one of the big challenges is the lack of high-quality imaging data in many of the large, federated databases being used to train these models. We may have the volume of patients, but without detailed imaging and nuanced clinical data, we are missing critical context that directly impacts outcomes. Take, for example, a patient with a nonhealing wound driven primarily by progressive diabetic neuropathy. Over time, neuropathy leads to loss of sympathetic tone, destabilizing the foot architecture. The bones begin to separate, leading to a Charcot foot. This alters pressure distribution, causing chronic compression in areas where wounds form. In that scenario, even if you improve perfusion, the microvascular supply remains compromised due to mechanical compression. AI needs to be able to account for these complex physiologic interactions, and this is where I think AI could eventually play a larger role, not just in identifying who might benefit from intervention, but in flagging cases where we might be doing harm. For instance, we have started to see data suggesting that in some claudicants, outcomes may actually worsen following intervention. That raises real concerns. Is the problem embolization? Is it lesion extension with each reintervention? Is it the cumulative burden of stents or other implants that bring their own set of issues? We are now seeing hints of a signal, especially over the last few years, that we need to question our assumptions. Every time we intervene, we are likely taking away some of the patient’s flow reserve. Looking back, I have been part of that pattern too, and I say this as someone who’s been involved in device development and intervention for decades. Sometimes, what we think we’re treating isn’t the real problem. And over time, through repetition and consensus, certain approaches become standard practice, even when their benefit isn’t as clear as we once believed. In CLTI, everyone seems to be taking their own approach to revascularization, but the broader trends suggest we may be headed in the wrong direction in some patient subsets. Something’s not adding up. I don’t pretend to have all the answers, but I do think it is time we start asking harder questions about lesion selection, patient selection, and how we define procedural success. Are we simply dealing with more diabetic patients, more advanced PAD, and more end-organ manifestations of the obesity epidemic? That could certainly be part of it. But what’s really driving the concern, at least from the conversations I’ve had with colleagues in vascular medicine, are the patterns emerging in claudication patients. There’s growing caution, and for good reason. When we start seeing signals that these patients might actually do worse after intervention, it forces us to re-examine what we are doing, and why.
At the same time, we are seeing utilization patterns in physician-owned and outpatient-based labs that are raising red flags.5 In some of these settings, atherectomy is being used in nearly 100% of cases. That prompts questions, especially when we know reimbursement incentives are different for atherectomy compared to other modalities. So now we have to ask: are these decisions always being driven by patient need, or are other forces at play? These are uncomfortable but necessary questions if we are serious about protecting patients and advancing the field.
Are there specific biomarkers or imaging modalities that you consider particularly useful in predicting procedural success or risk of amputation?
This is something we have been talking about for years. One of the big challenges is figuring out how to assess the burden of embolic debris and what impact it has downstream. Right now, we don’t have a reliable way to quantify microvascular injury or embolic load in the leg. There are experimental approaches, like using MRI to detect microinfarcts in skeletal muscle, especially in the calf, but none of that has been validated as a clinical surrogate for long-term outcomes like wound healing or limb salvage.
The biomarker side of this is equally interesting. We do know that the more intervention you do, let’s say when working through a long SFA occlusion, the more you often see a rise in systemic inflammatory markers afterward. So there is clearly some physiologic response to the work we are doing, almost like a localized “inflammatory storm.” But again, these markers are nonspecific, and no one’s really tracked them out to see whether they correlate with higher risk of amputation or worse long-term limb outcomes.
What we need is a tissue-specific biomarker or a reliable imaging-based surrogate for embolic burden and microvascular impact. If we had that, maybe we could better stratify risk before committing to extensive intervention. Combine that with AI-driven imaging data, and we might finally be able to identify which patients are truly at high risk for downstream harm. And maybe I’m wrong. Maybe some of that embolic debris is benign, and the body handles it just fine.
Do you think it’s worthwhile to image the outflow tract in advance to confirm it is patent before intervening, even if it adds cost to the workup?
Many don’t do meticulous imaging of the outflow before intervention because it is technically challenging and frankly, we don’t always want to know. But I think it’s critically important. When you do take the time to get both a pre- and post-procedure angiogram, you can actually see what vessels have been compromised. John Rundback talked about this in his review article.6
In my own practice, I have noticed two consistent patterns when I do this. First, you’ll almost always see some small vessel loss, maybe not major, but a branch here or there gets taken out. Second, when you have done a lot of work, you’ll start to see slow flow in the calf. That sluggish forward progression through the leg is something we’d recognize immediately in the heart. In cardiology, we score it using TIMI flow. If you open a coronary artery and see slow flow, that’s bad. It tells you there is significant downstream microvascular obstruction. But in the leg, we don’t have an equivalent system, and the limb doesn’t “speak” the same way.
If you embolize the brain, you know instantly. If you embolize the heart, you might get a delayed response depending on the extent, but you’ll likely see arrhythmias or hemodynamic instability. With the leg, it is silent. There is no obvious feedback, and that makes it easy to overlook. But if you’re seeing slow flow in the periphery after intervention, you may have already done more harm than you realize.
What we think is happening at the time of intervention and what we come to understand later through data and experience can be two very different things. In the peripheral space, I think we are still in the early stages of figuring out what the long-term consequences really are.
For the physician or cath lab team who might be reading this, what practical steps can they take to better protect their patients?
I truly believe that everyone doing these cases is in it for the right reasons: to help patients. And that includes not just the physician, but the entire cath lab team. The intent across the board is good. So if nothing else, I hope raising this concern increases awareness. Maybe it prompts someone to take a closer look at the runoff or the outflow tract. Maybe it sparks curiosity or even leads to new ideas for how we could quantify or better understand what is happening downstream.
This is still a hypothesis. But sometimes just identifying the problem is the most valuable first step. I remember a conversation I had years ago with a chief technology officer at an accelerator in California. He said, “Mark, stop trying to give me the solution. I don’t care what you think the fix is. Just give me the problem.” And that stuck with me. So that’s what I’m trying to do here: put a potential problem on the table and let smarter, younger, more creative people take it further. When we do that, we open the door for innovation. Maybe a new imaging protocol. Maybe a better distal protection strategy. Maybe a better way to identify patients who are particularly vulnerable to embolization. For example, when you see a long SFA occlusion with a lot of soft plaque and you are considering atherectomy, that’s when I personally believe you really need to consider using distal protection. I say that with no financial ties to protection devices or related technologies. That’s just my clinical experience talking.
I’m not here to push a specific solution without data. But if this conversation leads someone to explore the problem more rigorously, if it drives the kind of inquiry that results in real evidence five years from now, then that would be meaningful. That’s the kind of progress we need.
The comments presented herein reflect the opinions of Dr. Bates and do not represent the opinions of W.L. Gore, WVU, or the CAMC Institute of Academic Medicine.
References
1. Creager MA, Matsushita K, Arya S, et al. Reducing nontraumatic lower-extremity amputations by 20% by 2030: time to get to our feet: a policy statement from the American Heart Association. Circulation. 2021 Apr 27; 143(17): e875-e891. doi:10.1161/CIR.0000000000000967
2. Varcoe RL, DeRubertis BG, Kolluri R, et al; LIFE-BTK Investigators. Drug-eluting resorbable scaffold versus angioplasty for infrapopliteal artery disease. N Engl J Med. 2024 Jan 4; 390(1):9-19. doi:10.1056/NEJMoa2305637
3. Shammas NW, Dippel EJ, Coiner D, et al. Preventing lower extremity distal embolization using embolic filter protection: results of the PROTECT registry. J Endovasc Ther. 2008;15(3):270-276. doi:10.1583/08-2397.1
4. Narula N, Dannenberg AJ, Olin JW, et al. Pathology of peripheral artery disease in patients with critical limb ischemia. J Am Coll Cardiol. 2018 Oct 30; 72(18): 2152-2163. doi:10.1016/j.jacc.2018.08.002
5. Tsou TC, Dun C, Bose S, et al. Practice patterns of peripheral vascular interventions for peripheral artery disease in the office-based laboratory setting versus outpatient hospital. J Vasc Surg. 2024 Nov; 80(5): 1525-1536.e7. doi:10.1016/j.jvs.2024.06.006
6. Rundback J, Schneider PA, Fulton RE. Embolization during popliteal and tibial intervention for CLTI. Endovascular Today. 2022 May; 21(5): 57-61. https://assets.bmctoday.net/evtoday/pdfs/et0522_F6_Rundback.v2.pdf










