Conversations in Cardiology: The Late-Arriving STEMI Patient
© 2025 HMP Global. All Rights Reserved.
Any views and opinions expressed are those of the author(s) and/or participants and do not necessarily reflect the views, policy, or position of Cath Lab Digest or HMP Global, their employees, and affiliates.
Dr. Morton Kern with contributions from Drs. Eric Bates, Ann Arbor, Michigan; Jeffrey Cook, Quincy, Illinois; Kirk Garrett, Newport, Delaware; Steve Goldberg, Monterey, California; Tim Henry, Cincinnati, Ohio; J. Stephen Jenkins, New Orleans, Louisiana; Lloyd Klein, Sonoma, California; Paul Teirstein, La Jolla, California; Will Suh, Riverside, California.
Morton J. Kern, MD, MSCAI, FACC, FAHA
Clinical Editor; Interventional Cardiologist, Long Beach VA Medical Center, Long Beach, California; Professor of Medicine, University of California, Irvine Medical Center, Orange, California
Disclosures: Dr. Morton Kern reports he is a consultant for Abiomed, Abbott Vascular, Philips, ACIST Medical, and Opsens Inc.
Dr. Kern can be contacted at mortonkern2007@gmail.com
On X @MortonKern
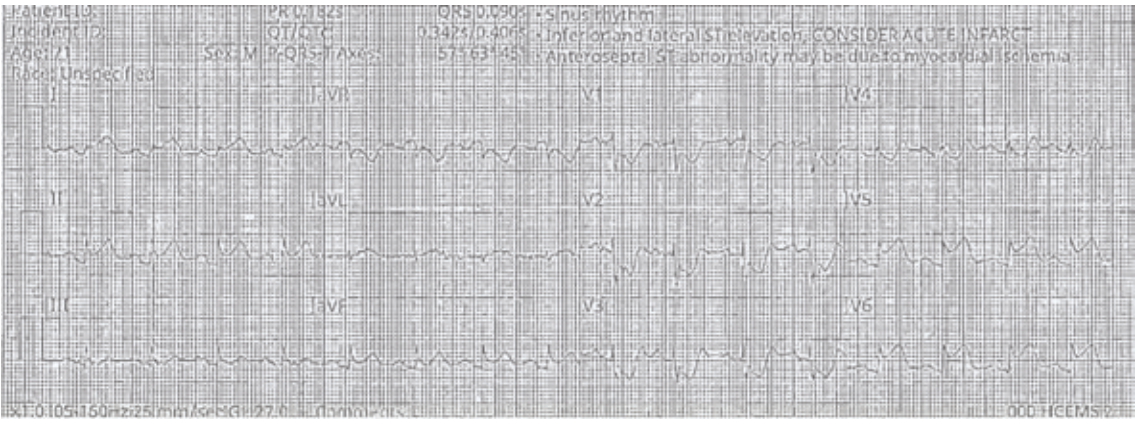
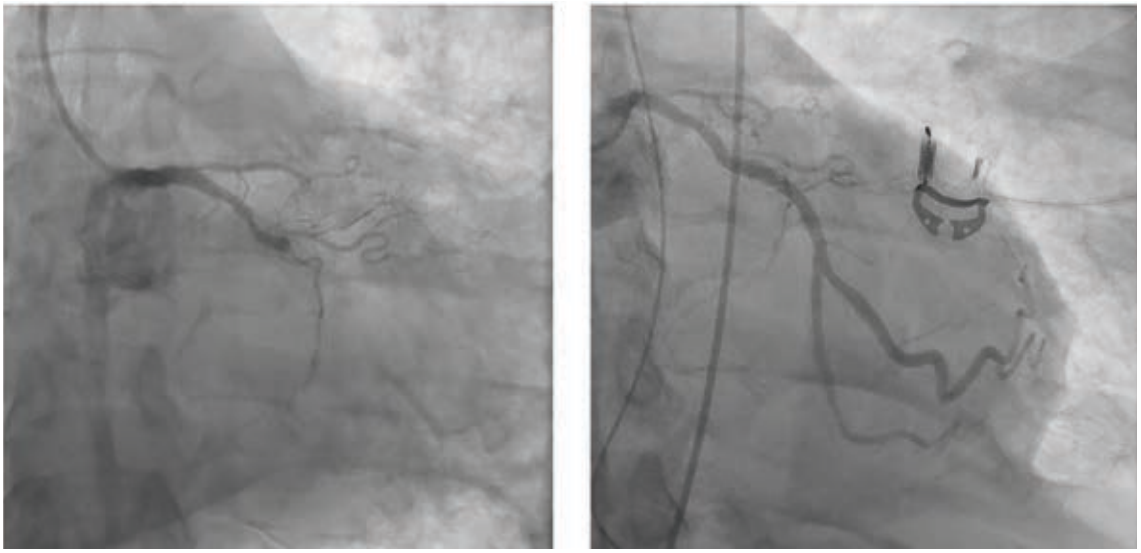
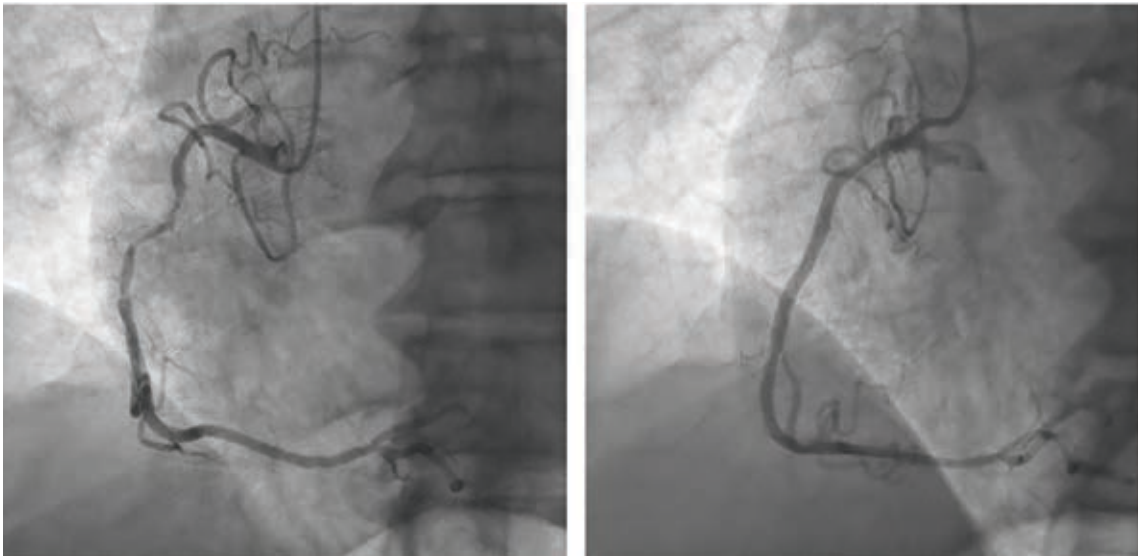
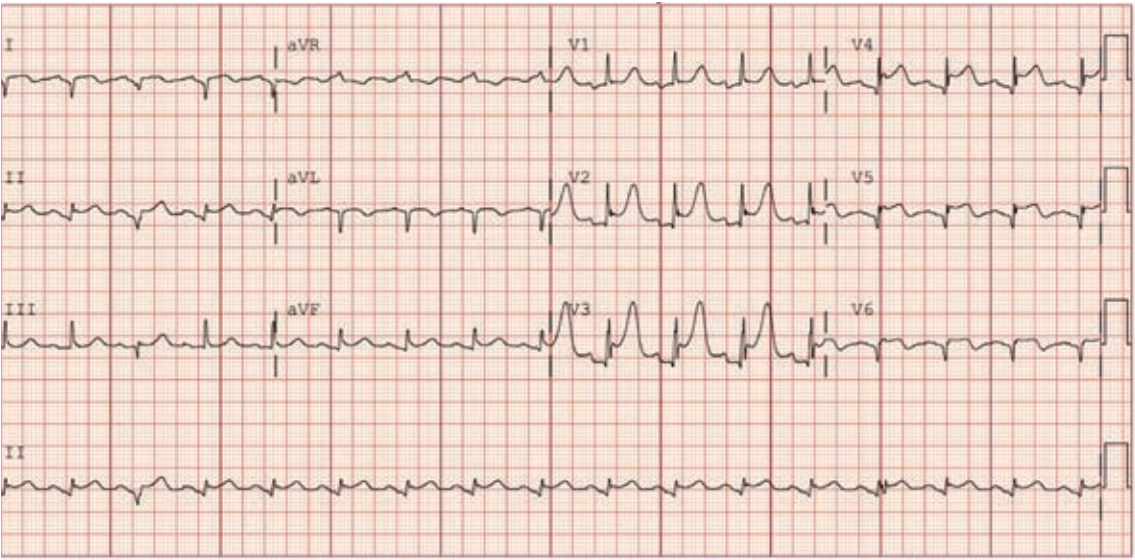
“Over the years as an interventionalist, writes Dr. Jeffrey Cook in Quincy, Illinois, “I have noticed a patient profile of an individual presenting somewhat late into their myocardial infarction (MI) — 12-24 hours or so — who seems to be overtly stable as they arrive at the emergency department (ED). Minimal pain, hemodynamics normal (or even hypertensive), oxygenation normal, no indication of decompensation noted. Everything seems perfectly routine. The patient is brought to the cath lab, the infarct-related artery is identified, wired, and ballooned — and from there, the patient decompensates. Even though the vessel has gone from 100% to 0%, suddenly the patient is hypotensive, with progressive respiratory distress, and progressing to shock. I have unfortunately been fooled by this presentation (Figures 1-4) many times, and I’m wondering a few things. First, what is the mechanism? Is it something to do with sudden washout of acidotic myocardium? Second, is there any way to predict that this is going to happen, and to take measures to prevent it? I recently had a patient present like this again, and I find myself extremely frustrated and distraught about it. Can you provide some guidance or direct me to appropriate literature? I’ve tried to find articles or even discuss it with other operators and have been stymied. I did find a thoughtful article by Yongbin Li et al,1 but it does not necessarily answer my questions.”
 Mort Kern, Long Beach, California: Dr. Cook, thanks for your question. The patient you described with a late-arriving (12-24h post) ST-elevation MI (STEMI) is a typical presentation and at increased risk for no-reflow and/or reperfusion injury, producing your exact clinical scenario. A number of interventionists and industry collaborators are working to identify and treat reperfusion injury with or without no reflow.
Mort Kern, Long Beach, California: Dr. Cook, thanks for your question. The patient you described with a late-arriving (12-24h post) ST-elevation MI (STEMI) is a typical presentation and at increased risk for no-reflow and/or reperfusion injury, producing your exact clinical scenario. A number of interventionists and industry collaborators are working to identify and treat reperfusion injury with or without no reflow.
To your specific questions:
(1) I do not know of a factor or sign that would predict reperfusion injury and/or no reflow, but the right coronary artery (RCA) seems to be more susceptible than the left anterior descending (LAD) and circumflex (CFX) arteries.
(2) The manner of re-opening the occluded vessel, fast and all at once, or slower with gradual smaller balloon inflations, favors the slower method. Fast, sudden reperfusion has been associated with no reflow, especially for the RCA (see Dr. James A. Goldstein’s work).
(3) Thrombus burden and duration of occlusion are directly related to the potential for reperfusion injury.
(4) Treatment often requires left ventricular support with drugs but is better with an intra-aortic balloon pump or if needed, Impella (Abiomed), depending on the severity of compromise and type of MI. My experience is that this period of hemodynamic compromise is short-lived and, depending on infarct size, survival is anticipated.
 Steve Goldberg, Monterey, California: Ironically, I had just such a patient a couple of days ago. The OAT (opening arteries late after MI) trial said that there was no benefit of percutaneous coronary intervention (PCI) if done 3-28 days after an MI, but did not address the common scenario of a patient presenting 1 to 3 days after their MI.2 I think it is common to open up those arteries, but it is unclear if it is helpful or not. I often find myself asking whether or not to open an artery when the MI presentation is delayed.
Steve Goldberg, Monterey, California: Ironically, I had just such a patient a couple of days ago. The OAT (opening arteries late after MI) trial said that there was no benefit of percutaneous coronary intervention (PCI) if done 3-28 days after an MI, but did not address the common scenario of a patient presenting 1 to 3 days after their MI.2 I think it is common to open up those arteries, but it is unclear if it is helpful or not. I often find myself asking whether or not to open an artery when the MI presentation is delayed.
 Paul Teirstein, La Jolla, California: Mort, the new acute coronary syndrome guidelines that were just updated and released about 10 days ago now have both aortic balloon pump and extracorporeal membrane oxygenation as class three recommendations for MI patients in shock, ie, don’t do it.3 Impella is the only mechanical circulatory support device that is recommended, if the peripheral vasculature can tolerate it. I’m not too sure [about the results], though; the number of patients in the trials is not very large and there are not many trials. Shock is tough. The outcomes are very iffy, so it is hard to show a difference. But the balloon pump is not something we’re supposed to be doing these days when patients are in shock. It’s hard for our age group [senior interventionalists] to believe mechanical support doesn’t help.
Paul Teirstein, La Jolla, California: Mort, the new acute coronary syndrome guidelines that were just updated and released about 10 days ago now have both aortic balloon pump and extracorporeal membrane oxygenation as class three recommendations for MI patients in shock, ie, don’t do it.3 Impella is the only mechanical circulatory support device that is recommended, if the peripheral vasculature can tolerate it. I’m not too sure [about the results], though; the number of patients in the trials is not very large and there are not many trials. Shock is tough. The outcomes are very iffy, so it is hard to show a difference. But the balloon pump is not something we’re supposed to be doing these days when patients are in shock. It’s hard for our age group [senior interventionalists] to believe mechanical support doesn’t help.
 Lloyd Klein, Sonoma, California: Hi Mort, this is clearly no reflow. Several studies show time of presentation after STEMI onset is predictive. Anyone can hypothesize why, but I don’t know if it’s been studied. The indication for emergency PCI in this setting (after 12 hours) is undefined.
Lloyd Klein, Sonoma, California: Hi Mort, this is clearly no reflow. Several studies show time of presentation after STEMI onset is predictive. Anyone can hypothesize why, but I don’t know if it’s been studied. The indication for emergency PCI in this setting (after 12 hours) is undefined.
 Tim Henry, Cincinnati, Ohio: All 4 sites in the Midwest STEMI Consortium (Minneapolis Heart Institute, Christ Hospital, Iowa Heart Center, Prairie Heart Institute) have always included 12-24 hours as our standard protocol and we recently published our data.4,5 In brief, the study includes 2 populations — a sicker group due to the late and larger infarcts, and a less sick group with borderline electrocardiogram (EKG) changes and intermittent symptoms. Regarding the specific case:
Tim Henry, Cincinnati, Ohio: All 4 sites in the Midwest STEMI Consortium (Minneapolis Heart Institute, Christ Hospital, Iowa Heart Center, Prairie Heart Institute) have always included 12-24 hours as our standard protocol and we recently published our data.4,5 In brief, the study includes 2 populations — a sicker group due to the late and larger infarcts, and a less sick group with borderline electrocardiogram (EKG) changes and intermittent symptoms. Regarding the specific case:
1. Overall, I think this is quite uncommon as long as you have adequate antiplatelet therapy on board. All 4 sites pretreat with ticagrelor and use cangrelor if the patient has cardiogenic shock or cardiac arrest.
2. Jay Traverse has data on a late-arriving cohort that had more microvascular obstruction and appears to benefit from supersaturated oxygen (SSO2) therapy.6 SSO2 therapy is something to strongly consider in the cohort with larger and late infarcts.
 J. Stephen Jenkins, New Orleans, Louisiana: The symptoms Dr. Cook’s patients are experiencing are demonstrating the Bezold-Jarisch reflex, a cardiovascular reflex involving the heart and nervous system. It’s triggered by certain stimuli that activate sensory receptors in the heart’s ventricles, resulting in a triad of effects: bradycardia, hypotension, and apnea or hypopnea. Though not described in the classic triad, nausea and vomiting are quite common. The reflex is mediated by cardiopulmonary chemoreceptors (primarily in the left ventricle) that send signals via the vagus nerve to the medulla. Activation of these receptors leads to a parasympathetic response with the hallmark triad above. It is much more common in inferior wall infarcts and worse the longer the vessel has been occluded. There is a simple way to stabilize your patient immediately that you should try next time: Reocclude the vessel with the PCI balloon and the response will immediately reverse! Wait 1 minute and reperfuse the territory again: the response will be delayed or not happen at all. Continue as long as necessary until you fool the medulla, just as we do with any of the denervation technologies that exist today.
J. Stephen Jenkins, New Orleans, Louisiana: The symptoms Dr. Cook’s patients are experiencing are demonstrating the Bezold-Jarisch reflex, a cardiovascular reflex involving the heart and nervous system. It’s triggered by certain stimuli that activate sensory receptors in the heart’s ventricles, resulting in a triad of effects: bradycardia, hypotension, and apnea or hypopnea. Though not described in the classic triad, nausea and vomiting are quite common. The reflex is mediated by cardiopulmonary chemoreceptors (primarily in the left ventricle) that send signals via the vagus nerve to the medulla. Activation of these receptors leads to a parasympathetic response with the hallmark triad above. It is much more common in inferior wall infarcts and worse the longer the vessel has been occluded. There is a simple way to stabilize your patient immediately that you should try next time: Reocclude the vessel with the PCI balloon and the response will immediately reverse! Wait 1 minute and reperfuse the territory again: the response will be delayed or not happen at all. Continue as long as necessary until you fool the medulla, just as we do with any of the denervation technologies that exist today.
 Eric Bates, Ann Arbor, Michigan: Let me offer a competing strategy to energize the conversation, assuming that this is reperfusion injury and not the Bezold-Jarisch reflex. Universal primary PCI up to 24 hours after MI probably represents overuse of the procedure. It is unlikely that acute benefit is gained in a minimally symptomatic patient with resolution of ST elevation. Acute benefit is more likely if the patients are still symptomatic with ischemic EKG changes or are having acute complications. The increased risk of the no-reflow phenomenon in latecomers may not matter (and is not prevented by antiplatelet therapy) if it is not associated with recurrent chest pain or ST segment elevation. However, the worst-case scenario is recurrent acute chest pain (usually worse than the initial discomfort), re-elevation of ST segments with acute and ugly T wave changes, contrast staining in the perfusion bed, and continued elevation of biomarker levels. I think this may represent hemorrhagic conversion in the infarct zone for some patients but seems to be the clinical correlate of reperfusion injury in the dog lab.
Eric Bates, Ann Arbor, Michigan: Let me offer a competing strategy to energize the conversation, assuming that this is reperfusion injury and not the Bezold-Jarisch reflex. Universal primary PCI up to 24 hours after MI probably represents overuse of the procedure. It is unlikely that acute benefit is gained in a minimally symptomatic patient with resolution of ST elevation. Acute benefit is more likely if the patients are still symptomatic with ischemic EKG changes or are having acute complications. The increased risk of the no-reflow phenomenon in latecomers may not matter (and is not prevented by antiplatelet therapy) if it is not associated with recurrent chest pain or ST segment elevation. However, the worst-case scenario is recurrent acute chest pain (usually worse than the initial discomfort), re-elevation of ST segments with acute and ugly T wave changes, contrast staining in the perfusion bed, and continued elevation of biomarker levels. I think this may represent hemorrhagic conversion in the infarct zone for some patients but seems to be the clinical correlate of reperfusion injury in the dog lab.
A competing strategy in asymptomatic patients is to wait a few days, do a perfusion study looking for residual ischemia in the infarct zone, and then perform a delayed angiogram with PCI of large, occluded infarct arteries with distal ischemia, not occluded side branch infarct arteries. The same benefit for late arrhythmia burden or LV remodeling can be achieved. Success rates are high, and complication rates are lower after the biochemical storm has passed. Complete revascularization of the non-infarct arteries can be achieved during the same procedure.
The FAST-MI report7 is quoted to support primary PCI in latecomers, but it included a selected population undergoing PCI 10-20 years ago, and may not have the same relevance now that every patient is undergoing angiography before hospital discharge and is being treated with complete revascularization. The surgeons learned to respect the acute infarct zone years ago, which is why they prefer to wait a few days before coronary artery bypass graft surgery in stable acute MI patients.
I suspect many reading this remember similar cases. The fact that Dr. Cook has had a few of these cases should prompt a re-evaluation of primary PCI for all latecomers in his lab. A selective acute strategy for latecomers with persistent symptoms, ischemic EKG changes, or electrical/hemodynamic instability might be more reasonable with angiography and complete revascularization for all before hospital discharge to minimize acute complications and promote long-term benefit.
 Kirk Garrett, Newport, Delaware: I believe both Tim and Steve are correct. I was taught that the Bezold-Jarisch reflex is mediated largely by parasympathetic afferents that are most abundant in the inferior and posterior walls, so infarcts involving those ventricular segments are most likely to trigger this reflex. However, sympathetic withdrawal is part of it, too, so the triad can be seen in MIs involving other myocardial beds.
Kirk Garrett, Newport, Delaware: I believe both Tim and Steve are correct. I was taught that the Bezold-Jarisch reflex is mediated largely by parasympathetic afferents that are most abundant in the inferior and posterior walls, so infarcts involving those ventricular segments are most likely to trigger this reflex. However, sympathetic withdrawal is part of it, too, so the triad can be seen in MIs involving other myocardial beds.
It’s worth remembering that the only Class 1 indication for PCI in stable STEMI patients with little/no pain is treatment within 12 hours of symptom onset.8 PCI in this setting is expected to lower mortality. PCI between 12-24 hours is a Class 2a indication with limited evidence (Level B, non-randomized). Here, PCI may improve aggregate clinical outcomes but isn’t proven to reduce mortality. Registry reports show a benefit, including late survival benefit, but in selected patients, those with lots of pain and EKG changes, still have salvageable myocardium, so PCI in these people should provide better results than conservative care. But for stable patients with little or no pain, the care plan outlined by Eric is the correct one.
Tim’s comment about SSO2 is interesting. I was part of SSO2 studies a long time ago and came away unconvinced — the track record is pretty spotty — but I’d support both PCI and promising, unproven treatments between 12-24 hours in the context of clinical studies.
 Will Suh, Riverside, California: The scenario that Dr. Cook describes sounds similar to what happens in a liver transplant in a DCD (donation after circulatory death) recipient. The liver transplant anesthesiologists at UCLA say it’s a scary roller coaster ride after the transplant surgeon unclamps the inferior vena cava. The hemodynamic instability is triggered by massive cytokine release from the ischemic graft after reperfusion. Re-occluding the infarct vessel might slow down the cytokine release and the vessel can then be reperfused more gradually.
Will Suh, Riverside, California: The scenario that Dr. Cook describes sounds similar to what happens in a liver transplant in a DCD (donation after circulatory death) recipient. The liver transplant anesthesiologists at UCLA say it’s a scary roller coaster ride after the transplant surgeon unclamps the inferior vena cava. The hemodynamic instability is triggered by massive cytokine release from the ischemic graft after reperfusion. Re-occluding the infarct vessel might slow down the cytokine release and the vessel can then be reperfused more gradually.
The Bottom Line
 Mort Kern, Long Beach, California: Dr. Cook’s patient likely had reperfusion injury with or without no reflow. The worst scenario is one with recurrent pain and ST elevation with an open artery. No reflow with ST elevation usually is self-limited with supportive care. If the same patient is in cardiogenic shock, then your approach should proceed along the recent guideline released this year for the management of shock. I thank Dr. Cook and my colleagues for another stimulating conversation in cardiology.
Mort Kern, Long Beach, California: Dr. Cook’s patient likely had reperfusion injury with or without no reflow. The worst scenario is one with recurrent pain and ST elevation with an open artery. No reflow with ST elevation usually is self-limited with supportive care. If the same patient is in cardiogenic shock, then your approach should proceed along the recent guideline released this year for the management of shock. I thank Dr. Cook and my colleagues for another stimulating conversation in cardiology.
References
1. Yao Z, Li W, Cheng L, Cao M, Pang Z, Li Y. Comparison of the effect of recombinant human pro-urokinase and tirofiban on myocardial blood flow perfusion in ST elevation myocardial infarction patients receiving primary percutaneous coronary intervention: A one-center retrospective observational study. Medicine (Baltimore). 2019 Jul; 98(27): e16143. doi:10.1097/MD.0000000000016143
2. Kruk M, Kadziela J, Reynolds HR, et al. Predictors of outcome and the lack of effect of percutaneous coronary intervention across the risk strata in patients with persistent total occlusion after myocardial infarction: Results from the OAT (Occluded Artery Trial) study. JACC Cardiovasc Interv. 2008 Oct;1(5):511-20. doi:10.1016/j.jcin.2008.08.007
3. Rao SV, O’Donoghue ML, Ruel M, et al. 2025 ACC/AHA/ACEP/NAEMSP/SCAI guideline for the management of patients with acute coronary syndromes: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2025 Apr; 151(13): e771-e862. doi:10.1161/CIR.0000000000001309.
4. Griffin ACG, Yildiz M, Bradley S, et al. Frequency and outcomes of STEMI patients presenting between 12 and 24 h after symptom onset: Late-presenting STEMI. Catheter Cardiovasc Interv. 2023 Jan;101(1):1-10. doi:10.1002/ccd.30495
5. Yildiz M, Sharkey S, Aguirre FV, et al. The Midwest ST-Elevation Myocardial Infarction Consortium: design and rationale. Cardiovasc Revasc Med. 2021 Feb; 23: 86-90. doi:10.1016/j.carrev.2020.08.019
6. Carlson PE, Schwager S, Feldewerd K, Schmidt S, Campbell M, Bergstedt S, Cavalcante JL, Traverse JH. The use of supersaturated oxygen therapy in patients with late-presentation anterior ST-segment elevation myocardial infarction. Int J Cardiol. 2025 Jul 15; 431:133256. doi:10.1016/j.ijcard.2025.133256
7. Bouisset F, Gerbaud E, Bataille V, et al; FAST-MI Investigators. Percutaneous myocardial revascularization in late-presenting patients with STEMI. J Am Coll Cardiol. 2021 Sep 28; 78(13): 1291-1305. https://www.jacc.org/doi/10.1016/j.jacc.2021.07.039
8. Writing Committee Members; Lawton JS, Tamis-Holland JE, Bangalore S, et al. 2021 ACC/AHA/SCAI guideline for coronary artery revascularization: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2022 Jan 18; 79(2): e21-e129. doi:10.1016/j.jacc.2021.09.006










