Air Embolus in the Cardiac Catheterization Laboratory – A Highly Preventable Procedural Complication
© 2025 HMP Global. All Rights Reserved.
Any views and opinions expressed are those of the author(s) and/or participants and do not necessarily reflect the views, policy, or position of Cath Lab Digest or HMP Global, their employees, and affiliates.
Morton J. Kern, MD, MSCAI, FACC, FAHA
Clinical Editor; Interventional Cardiologist, Long Beach VA Medical Center, Long Beach, California; Professor of Medicine, University of California, Irvine Medical Center, Orange, California
Disclosures: Dr. Morton Kern reports he is a consultant for Abiomed, Abbott Vascular, Philips, ACIST Medical, and Opsens Inc.
Dr. Kern can be contacted at mortonkern2007@gmail.com
On X @MortonKern
Editor's Note: An addendum by Dr. Kern was placed at the end of this article on August 11th regarding air embolism in left atrial appendage occlusion procedures.
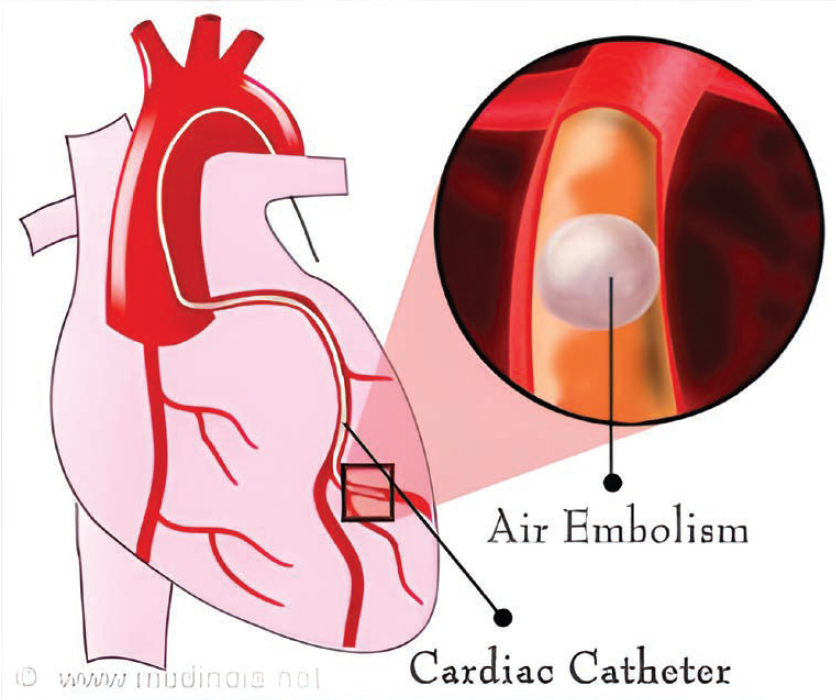
Reprinted from DMello S. Coronary artery air embolism, a dreaded complication, now has a cure. Medindia. May 2015. Accessed July 16, 2025. https://www.medindia.net/news/healthinfocus/coronary-artery-air-embolism-a-dreaded-complication-now-has-a-cure-149399-1.htm#google_vignette
I was recently asked to review a case of a patient who had a myocardial infarction after a coronary angiogram, caused by an air embolus. The patient recovered, but I realized I had incomplete knowledge of this complication. I thought I’d review the subject here for our cath lab colleagues.
Air embolisms (AEs) occur when air enters or traverses a vessel or chamber. Air can enter either into the venous or arterial circulation, moving along a pressure gradient or traveling with gravity. AEs are usually iatrogenic (as opposed to gas formed during some infections) and are frequently associated with various invasive procedures or interventions (Table 1). AEs can transiently obstruct the coronary blood flow, producing ischemia (Figure 1), typically detected during or immediately after a procedure by symptoms such as chest pain, dyspnea, confusion, or altered consciousness (eg, encephalopathy). AEs may also be silent and be detected incidentally on repeat imaging. The most common AEs occur during central venous catheterization. With central venous pressure catheter placement, an air embolus may occur in approximately 0.2-1% of patients.1 Treatment may prove futile if the air bolus is >50 ml.1
 In the cath lab, the most common occurrence of an air embolus results from inadvertent injection of air into a coronary artery through the manifold, tubing, and coronary catheter. An air embolus can also occur during any large-bore procedure, or even during flushing of intravascular imaging or aspiration catheters. Air bubbles may be injected if there is insufficient aspiration of blood during the clearing of the catheter or with poor flushing techniques. Rarely, an AE can occur during device exchanges or loose port connections where removal of a large catheter creates a transient vacuum and air enters the guide catheter. Iatrogenic introduction of air during catheter manipulation or exchange is rare and usually harmless (1 in 3000 cases).2
In the cath lab, the most common occurrence of an air embolus results from inadvertent injection of air into a coronary artery through the manifold, tubing, and coronary catheter. An air embolus can also occur during any large-bore procedure, or even during flushing of intravascular imaging or aspiration catheters. Air bubbles may be injected if there is insufficient aspiration of blood during the clearing of the catheter or with poor flushing techniques. Rarely, an AE can occur during device exchanges or loose port connections where removal of a large catheter creates a transient vacuum and air enters the guide catheter. Iatrogenic introduction of air during catheter manipulation or exchange is rare and usually harmless (1 in 3000 cases).2
Massive air embolism in cardiac bypass procedures is rare, occurring in between 0.003% and 0.007% of cases, with 50% having adverse outcomes.1 AE is most associated with otolaryngology and neurosurgical procedures due to the location of the surgical incision. The sitting position in posterior craniotomies is deemed especially risky and procedure-related complications of venous air have been estimated to be between 10% and 80%.3 This fact should be kept in mind for procedures in the cath lab when the patient’s head is in an elevated position.
While air in the coronary arteries may impede coronary flow producing ischemia, air in the left ventricle impedes diastolic filling. During systole, air is pumped into the coronary arteries, disrupting coronary perfusion, and leading to acute hypoxemia and hypercapnia. Air may produce acute changes in right ventricular (RV) pressure, resulting in RV strain, which can lead to right heart failure, decreased cardiac output, RV ischemia, and arrhythmia, followed by systemic circulatory collapse and even death.

On the venous side, great caution should be exercised in patients with a suspected patent foramen ovale (PFO). Paradoxical venous embolism in patients with PFO/shunts can produce a devastating stroke. The risk of venous air embolus is increased by low central venous pressure or inspiration, reducing intrathoracic pressure below atmospheric pressure.
Mechanisms of Symptoms
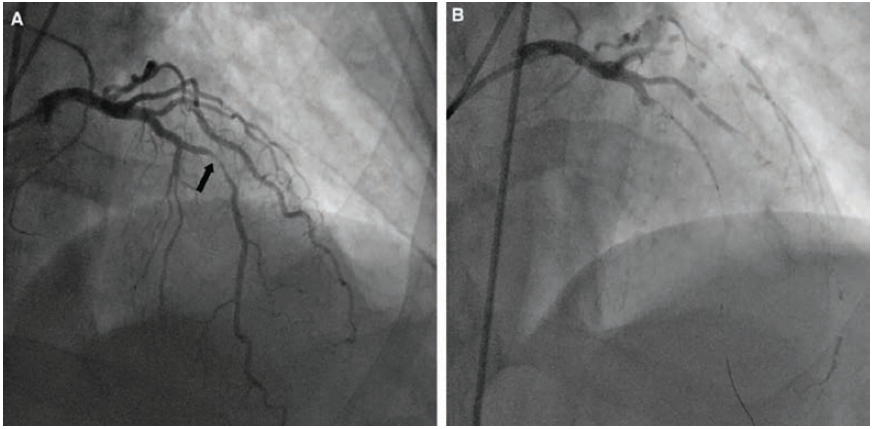
Reprinted with permission from Prasad A, Banerjee S, Brilakis ES. Images in cardiovascular medicine. Hemodynamic consequences of massive coronary air embolism. Circulation. 2007 Jan 30; 115(4): e51-53. doi:10.1161/CIRCULATIONAHA.106.655977
A coronary air embolism can be seen on angiography as a lucent spherical or circular lucency within the contrast-filled vessel or structure (Figures 2-3). The sequelae and pathophysiology of AEs arise from obstruction to blood flow, ischemia, and infarction from air entering the coronary arteries, brain, or other end organs resulting in reduced tissue perfusion. The lethal volumes of air in an acute bolus is approximately 0.5–0.75 ml/kg in rabbits and 7.5–15.0 ml/kg in dogs.4 The lethal dose for humans is theorized to be 3-5 ml/kg, estimated at 300-500 ml of gas introduced at a rate of 100 ml/sec.4 Air infusion rates of more than 1.5ml/kg/min are associated with bradycardia and cardiovascular decompensation.3 The rate of accumulation and patient position contribute to the lethality.4
Reprinted with permission from Chuang DY, Sundararajan S, Sundararajan VA, et al. Accidental air embolism. Stroke. 2019 Jul; 50(7): e183-e186. doi:10.1161/STROKEAHA.119.025340
Diagnosis of Air Embolus
Prompt recognition and team coordination are critical. Early diagnosis depends on an index of suspicion for new clinical signs which may include sudden hypotension, bradycardia or tachycardia, chest pain or ST changes or seizures, and focal neurologic deficits. Cardiac arrest has been reported in rare and severe cases. Diagnostic modalities beyond angiographic or computed tomography angiography include Doppler ultrasonography, a sensitive and practical means of detecting intracardiac air, often used during neurosurgical procedures with the patient in a sitting position. Transesophageal echocardiography is more sensitive than Doppler and is frequently utilized by anesthesiologists to monitor patients during high-risk procedures.
Management of Air Embolus
Coronary air embolus is only one of the many potential complications related to coronary angiography. Table 2 lists some of the coronary and peripheral vascular complications and their management strategies. Treatment starts by stopping air entry into the system, followed by several other interventions such as Trendelenburg position and left lateral decubitus position (also known as Durant’s maneuver).5
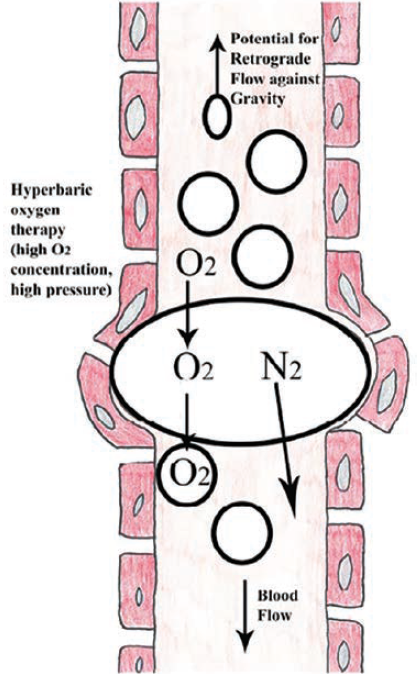
Figure 4 illustrates how positioning and gravity can influence the movement of air against the blood flow direction and reduce the impact of embolic occlusion. Air in the heart can be stabilized within the left ventricular (LV)/RV apex. Left lateral decubitus positioning allows air to move toward the RV apex, thereby relieving the obstruction of the pulmonary outflow tract. Another method is to aspirate air via a guiding catheter.6 A microcatheter may be able to reach more distally than a guide catheter. Some operators have suggested a forceful injection of saline or contrast to disperse air.2
 Patients with an air embolus should receive 100% oxygen administration, which works by reducing the nitrogen content in the bubbles shrinking the bubble size. Air is mostly composed of nitrogen (78%), oxygen (21%), argon (1%), and carbon dioxide (0.04%).7 Replacing nitrogen with O2 at the higher concentration of O2 helps reduce bubble obstruction.
Patients with an air embolus should receive 100% oxygen administration, which works by reducing the nitrogen content in the bubbles shrinking the bubble size. Air is mostly composed of nitrogen (78%), oxygen (21%), argon (1%), and carbon dioxide (0.04%).7 Replacing nitrogen with O2 at the higher concentration of O2 helps reduce bubble obstruction.
Hyperbaric oxygen therapy (HBOT) should be considered early in severe cases of air embolus (Figure 5). HBOT has been used for years to treat divers who have rapidly ascended after prolonged exposure to deep ocean pressure. Under deep diving pressure exposure, air in the diver’s inspired gases enters the blood and is compressed. On rapid decompression, the bubbles come out of solution and can block blood flow to various vital organs, but particularly the brain with central nervous system complications (decompression sickness, or the bends). For these patients, hyperbaric oxygen administration is the primary therapy.
Additionally, ventilation with 100% O2 corrects hypoxemia and increases the diffusion gradient for nitrogen out of the bubbles, causing them to shrink.8 HBO administration reduces bubble size due to the absorption of nitrogen from the bubble. Elevated ambient pressure reduces the bubble size in accordance with Boyle’s law. At 282 kPa (kilopascal), a conventional HBO treatment pressure, gas bubble diameter will be reduced to 82%, a 45% decrease in volume, promoting passage through the microcirculation and resolution of embolic phenomena.8
In a review of a case series with 27 patients, Edsell et al9 noted a substantial improvement in outcomes was shown in patients treated with HBO. Of these patients, 346/441 (78%) who received HBO fully recovered and 20 (4.5%) died. Of the 288 with no recompression therapy, 74 (26%) fully recovered and 151 (52%) died.
Treating Coronary Slow/No Flow
Although thought to be mostly ineffective for air bubbles, therapies for coronary slow/no flow can be given during the institution of measures to stabilize blood pressure and any resuscitative attempts that may be required. First-line management includes intracoronary administration of adenosine (10-20 mcg bolus), verapamil (100-200 mcg bolus), and nitroprusside (50-100 mcg bolus). Alternative agents also thought to be effective include diltiazem (0.5-2.5 mg), papaverine (10-20 mcg), nicardipine (200 mcg), nicorandil (2 mcg), and epinephrine (50-100 mcg). Ineffective or contraindicated treatments for no reflow related to air embolus include intracoronary nitroglycerin, coronary artery bypass graft surgery, stent placement, and thrombolytics.
Innovations and Prevention
Staff education and air embolism checklists are an important starting point for lab improvement and protection. All personnel dealing with tubing, connectors, Luer-lock syringes, and air-eliminating devices should ensure tight connections and correct functioning. Do not attempt to bypass or silence bubble detectors. Inline microbubble detectors in bypass circuits and in the ACIST CVi Contrast Delivery System are designed as critical life-protecting implementations. For a quick, accurate assessment, operators can use real-time intracardiac echocardiography imaging to detect intravascular air.10,11
The Bottom Line
Air embolism is a preventable but serious complication. Early recognition and intervention reduce morbidity and mortality. Continued innovation in system protocols, equipment security for connections and leaks, with frequent checks on technique, and early-detection diagnostic tools will reduce complications and adverse outcomes.
Addendum
It was quite fortuitous that our recent CLD editor’s corner was devoted to air embolism (AE) (August 2025) as I read the FDA warning summary of air embolism risk with the Watchman left atrial appendage occlusion (LAAO) access system. As reported by Todd Neale in the August 06, 2025, TCTMD page, as of July 30, there have been 120 serious injuries and 17 deaths related to AE. AE risk was limited only to the implant technique. In a review of their clinical events, Boston Scientific updated the instructions for use to minimize AE. Air is more likely to get in when the procedure is performed without positive pressure-controlled ventilation.
To recap some of the discussion surrounding AE in CLD, negative intracavity pressure relative to atmosphere pressure sets the stage of air to enter the body, cavity or vessel. The risk of negative left atrium pressure and air entry is considerably higher during transseptal access in patients under conscious or deep sedation. Preexisting low left atrial pressure, hypovolemia, and partial upper airway collapse predispose to AE.
For coronary AE, and now LAAO AE, the most common manifestation of air embolism is ST-segment elevation or visible air bubbles on imaging. As with coronary angiographic AE, the LAAO AE mostly were self-limited or required only temporary medical intervention. A Boston Scientific spokesperson indicated that “Based on the data included in this investigation on all Watchman cases performed between September 2024-May 2025, we estimate the rate of death resulting from air embolism to be 0.009%.”
Todd Neale spoke with Dr. Andrew Goldsweig, lead author of new multisociety LAAO guidelines, who reemphasized my thoughts on AE and how critical it is to remove air from all intravascular catheters and devices. Dr. Goldsweig also notes, “There is nothing Watchman- or LAAO-specific to this warning: the same issue applies to all transseptal catheterization procedures.” I was gratified and agreed wholeheartedly with this teaching and recommendation.
— Mort Kern
References
1. Hammon JW, Hines MH. Extracorporeal Circulation. In: Cohn LH, editor. Cardiac Surgery in the Adult. 4th ed. New York: McGraw-Hill; 2012: Chapter 12.1.
2. Khan M, Schmidt DH, Bajwa T, Shalev Y. Coronary air embolism: incidence, severity, and suggested approaches to treatment. Cathet Cardiovasc Diagn. 1995 Dec; 36(4): 313-318. doi:10.1002/ccd.1810360406
3. Palmon SC, Moore LE, Lundberg J, Toung T. Venous air embolism: A review. J Clin Anesth. 1997;9:251-57. doi:10.1016/s0952-8180(97)00024-x
4. Mirski MA, Lele AV, Fitzsimmons L, Toung TJ. Diagnosis and treatment of vascular air embolism. Anesthesiology. 2007; 106: 164-177. doi:10.1097/00000542-200701000-00026
5. Suastika LO, Oktaviono YH. Multiple air embolism during coronary angiography: how do we deal with it? Clin Med Insights Cardiol. 2016 May 19; 10: 67-70. doi:10.4137/CMC.S38040
6. Patterson MS, Kiemeneij F. Coronary air embolism treated with aspiration catheter. Heart. 2005 May; 91(5): e36. doi:10.1136/hrt.2005.060129
7. Earth’s atmosphere composition: nitrogen, oxygen, argon and CO2. EarthHow. https://earthhow.com/earth-atmosphere-composition/. Accessed July 21, 2025.
8. Tovar EA, Del Campo C, Borsari A, et al. Postoperative management of cerebral air embolism: gas physiology for surgeons. Ann Thorac Surg. 1995 Oct; 60(4): 1138-1142. doi:10.1016/0003-4975(95)00531-o
9. Edsell ME, Kirk-Bayley J. Hyperbaric oxygen therapy for arterial gas embolism. Br J Anaesth. 2009 Aug; 103(2): 306; author reply 306-307. doi:10.1093/bja/aep186
10. McCarthy CJ, Behravesh S, Naidu SG, Oklu R. Air Embolism: diagnosis, clinical management and outcomes. Diagnostics (Basel). 2017 Jan 17; 7(1): 5. doi:10.3390/diagnostics7010005
11. Berg R, Lim MJ. Complications of percutaneous coronary intervention. In: Kern MJ, ed. The Interventional Cardiac Catheterization Handbook. 3rd ed. Philadelphia: Saunders; 2012:122.










