Aligning Innovation with Access: A Physician’s Guide to Agent Drug-Coated Balloon Reimbursement
© 2025 HMP Global. All Rights Reserved.
Any views and opinions expressed are those of the author(s) and/or participants and do not necessarily reflect the views, policy, or position of Cath Lab Digest or HMP Global, their employees, and affiliates.
Partha Sardar, MD
Division of Cardiology, Columbia University Irving Medical Center/ New York-Presbyterian Hospital, New York, New York
Disclosures: Dr. Partha Sardar reports no conflicts of interest regarding the content herein.
Partha Sardar, MD, can be contacted at psardar24@gsb.columbia.edu.
The drug-coated balloon (DCB) marks a pivotal advancement in the treatment of in-stent restenosis. As the first and only FDA-approved coronary DCB in the United States, the Agent DCB (Boston Scientific) offers a stent-free approach to percutaneous coronary intervention (PCI), delivering paclitaxel directly to the vessel wall to inhibit neointimal hyperplasia and support long-term vessel patency.1,2 However, with innovation comes complexity, particularly in the reimbursement landscape. Physicians and hospitals must navigate evolving coding structures, transitional payment models, and payer-specific guidelines to ensure appropriate reimbursement. The relatively higher upfront cost of the Agent DCB, particularly in an era of declining reimbursements and increasing scrutiny of healthcare spending, poses challenges for widespread adoption.
This guide is intended to help physicians understand appropriate patient selection, reimbursement pathways, documentation requirements, and best practices that support sustainable use and value-driven adoption of the DCB.
Patient Selection: Identifying the Right Candidates
Inappropriate or indiscriminate use of advanced technologies can increase healthcare costs without commensurate clinical benefit. Therefore, patient selection should be informed by data from pivotal trials and regulatory guidance. The Agent DCB received FDA approval in the United States based on the pivotal AGENT IDE trial.1 Until additional real-world data or trial results become available, the inclusion and exclusion criteria from this study serve as the best guidance for patient selection.
Eligible patients in the AGENT IDE trial had in-stent restenosis with a reference vessel diameter between 2.0 mm and 4.0 mm, and a lesion length of 26 mm or less. Target lesion stenosis had to be greater than 50% in symptomatic patients or greater than 70% in those without symptoms.
Patients were excluded if they had a recent ST-elevation or Q-wave myocardial infarction, unprotected left main disease, saphenous vein grafts or arterial bypass graft disease, left ventricular ejection fraction below 25%, or thrombus in the target vessel. Additionally, there is currently inadequate supporting data for the use of the Agent DCB in chronic total occlusions or lesions requiring treatment with more than one balloon.
Most Appropriate Use Cases
The Agent DCB can be particularly useful in in-stent restenosis cases where additional stenting would be suboptimal, eg, bifurcation lesions or patients with multiple existing stent layers. In the AGENT IDE trial, over 40% of treated lesions had ≥2 stent layers, and half involved diffuse in-stent restenosis, scenarios where the Agent DCB demonstrated a 10.7% absolute risk reduction in target lesion failure.2
Cost of the Agent DCB
In the United States, the average sales price for Agent DCB is approximately $5,000–$6,000 per unit.3 Cost concerns can be a barrier to adoption unless appropriate reimbursement is secured. Physicians should work closely with hospital billing and reimbursement teams to ensure coding accuracy and optimal payer support.
Outpatient Reimbursement Pathways
New Coding and Payment Framework (Effective January 1, 2025)
To encourage the adoption of innovative technologies, the Centers for Medicare & Medicaid Services (CMS) has approved a Transitional Pass-Through (TPT) payment for coronary DCBs, recognizing their clinical advancement. Under the Hospital Outpatient Prospective Payment System (OPPS), Medicare reimburses based on Ambulatory Payment Classifications (APCs). The Agent DCB qualifies for separate TPT reimbursement when used in eligible outpatient cases.4,5
 In-Stent Restenosis Diagnosis Coding
In-Stent Restenosis Diagnosis Coding
The Agent DCB is FDA-approved for treating in-stent restenosis. Use the ICD-10- CM code T82.855A (Stenosis of coronary artery stent, initial encounter) when billing Agent DCB procedures for in-stent restenosis. This code can be used as the primary or secondary diagnosis. If restenosis is due to disease progression, assign the underlying disease as the primary diagnosis and T82.855A as secondary, per coding guidelines.
New Category III CPT Codes
Two new CPT codes have been introduced:
0913T – Use for standalone DCB interventions in a single major coronary artery.
+0914T – Use in conjunction with another PCI procedure when DCB is performed on a separate target lesion.
These codes are essential for capturing DCB-related procedures accurately. Use of unlisted CPT codes is discouraged now that specific codes exist.
Understanding Category III CPT Codes
Category III codes are temporary codes used to track emerging technologies. They allow data collection but are not assigned a relative value units (RVUs) by CMS. Reimbursement depends on individual Medicare Administrative Contractors (MACs) and private payers.
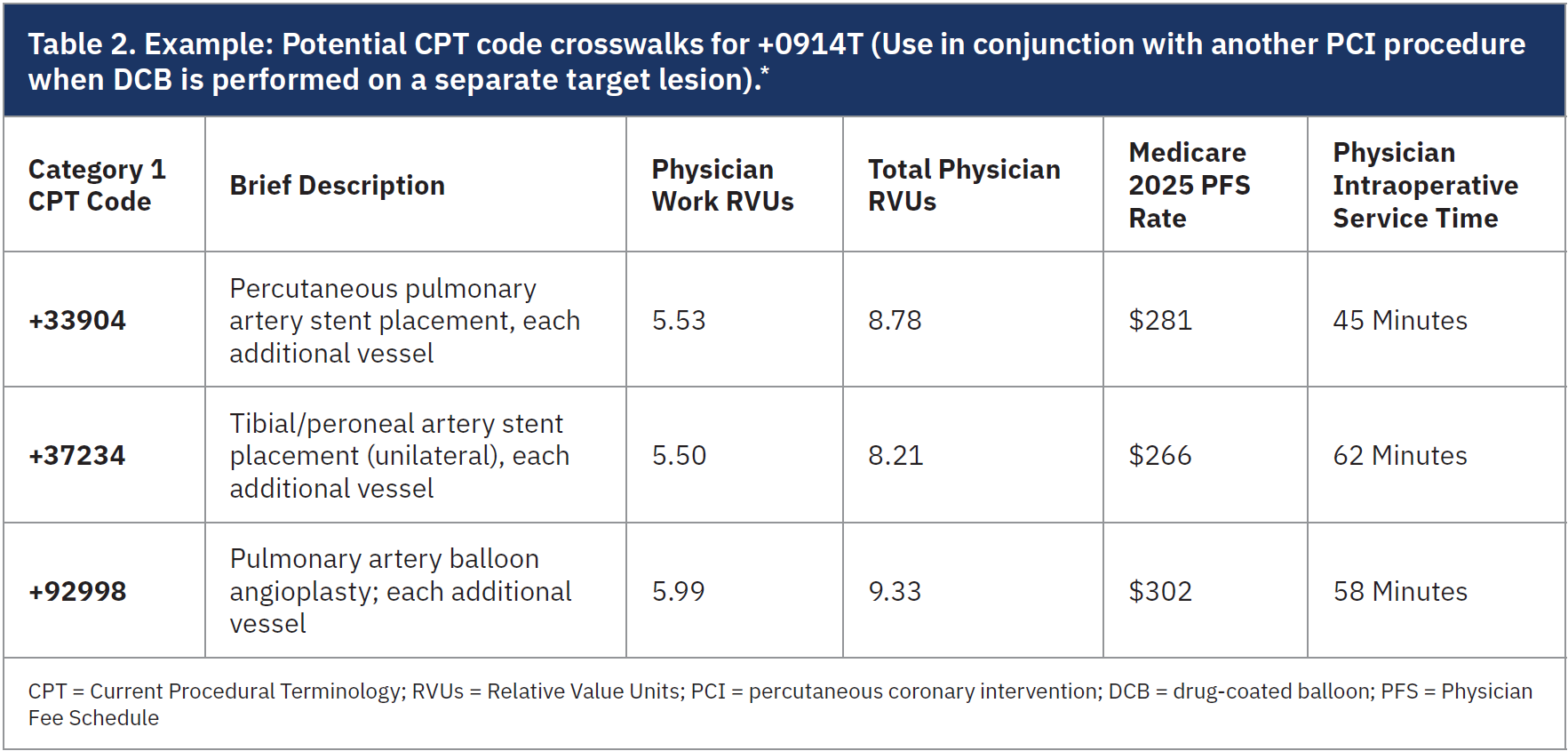
en-US/reimbursement/interventional-cardiology.html). Providers should review Boston Scientific team’s disclaimer on these recommendations and consult their own revenue cycle teams to determine the most appropriate CPT code crosswalks for 0913T and +0914T, based on how these codes align with their clinical practice.6
Physician Reimbursement Considerations
Crosswalk Methodology
Since the Category III code has not been assigned RVUs by CMS, physicians and institutional revenue cycle teams should “crosswalk” it to a comparable Category I CPT code based on procedural complexity and resource utilization, such as drug-eluting stent placement, as a reference for determining reimbursement (Tables 1-2).3
C-Code Reporting
The new HCPCS code C9610 (Catheter, transluminal drug delivery with or without angioplasty, coronary, non-laser; insertable) describes the Agent DCB and is reported by facilities for outpatient procedures. C9610 qualifies for Medicare’s TPT payment. To receive this additional reimbursement, hospitals must report C9610 along with the appropriate CPT/HCPCS procedure code on outpatient claims.6
Inpatient Payment Pathways
For hospital inpatient stays, Medicare reimburses under the Inpatient Prospective Payment System (IPPS), with payment determined by the assigned Medicare Severity- Diagnosis Related Group (MS-DRG). This is influenced by the principal diagnosis, such as in-stent restenosis or coronary artery disease, as well as comorbidities like heart failure and procedural complexity. Accurate and specific documentation of the patient’s clinical conditions and diagnoses is critical, as it directly impacts reimbursement.
ICD-10-PCS Coding for Coronary DCB
Effective October 1, 2024, 16 new ICD-10- PCS codes were introduced for coronary DCB procedures. These codes must be reported alongside PCI procedure codes describing lesion preparation methods (eg, angioplasty or atherectomy). The most commonly used code for coronary DCB is XW0J3HA – Introduction of paclitaxel-coated balloon technology, one balloon into coronary artery, one artery. The remaining 15 codes follow the format +XW0_3_A, depending on the number of balloons used and arteries treated.
Major Complications (MCCs)
A Major Complication or Comorbidity (MCC) indicates a higher resource need and influences MS-DRG assignment. Proper documentation of MCCs supports appropriate inpatient reimbursement. While MCCs are critical for Medicare, commercial payers may not follow the same guidelines.
 Documentation Suggestions
Documentation Suggestions
Thorough documentation is essential not only for appropriate reimbursement, but also to support compliance and protect against audits. Key elements include:
(1) A clearly documented diagnosis of in-stent restenosis confirmed by angiographic evidence;
(2) Justification for DCB use based on clinical factors such as prior stent failure;
(3) Detailed procedural notes outlining vessel preparation, balloon sizing, each distinct lesion treated, and any adjunctive treatments used;
(4) Documentation should also indicate previous therapies and the rationale for selecting a drug-coated balloon as the next line of treatment.
When reporting Category III CPT codes, providers should follow a well-defined strategy. This includes verifying payer-specific billing requirements, documenting medical necessity, and obtaining prior authorization where required. CPT code 0913T should be reported per encounter, often with a crosswalk to a comparable Category I CPT code for context. Submitting a letter of explanation along with estimates for RVUs and resource comparisons can support the claim.
Commercial Payer Considerations
Coverage for Category III CPT codes among commercial payers can vary significantly. While some private insurers follow Medicare policy and recognize the codes with comparable reimbursement structures, others require individual negotiation or may not yet have established clear coverage policies. Prior authorization is often necessary, and thorough documentation of medical necessity is essential to prevent denials. Additionally, reimbursement practices may differ; some payers treat the device as a separately billable item, while others may bundle it into the overall procedural cost. Understanding the specific policies of each payer is crucial to ensure adequate reimbursement (Summary Table).
Conclusion
The Agent DCB provides a transformative approach to the management of in-stent restenosis. While its clinical benefits are well-supported, high acquisition costs and reimbursement complexity demand a thoughtful strategy for successful implementation.
By focusing on evidence-based patient selection, understanding Medicare and commercial payer pathways, using new Category III CPT codes, and ensuring thorough documentation, physicians can support appropriate and sustainable adoption of this novel technology, ultimately improving outcomes for patients with coronary in-stent restenosis.
References
1. Yeh RW, Shlofmitz R, Moses J, et al; AGENT IDE Investigators. Paclitaxel-coated balloon vs uncoated balloon for coronary in-stent restenosis: the AGENT IDE randomized clinical trial. JAMA. 2024; 331(12): 1015-1024. https://jamanetwork.com/journals/jama/fullarticle/2816073
2. Kundu A, Moliterno DJ. Drug-coated balloons for in-stent restenosis-finally leaving nothing behind for US patients. JAMA. 2024; 331(12): 1011-1012. https://jamanetwork.com/journals/jama/fullarticle/2816074
3. Medicare Electronic Application Request Information System (MEARiS). AGENT Paclitaxel-Coated Balloon Catheter - DEP2402295H2TU - CY 2025 Q4. Accessed May 4, 2025. Available at: https://mearis.cms.gov/public/publications/device-ptp/DEP2402295H2TU.
4. Centers for Medicare & Medicaid. CY2025 Physician Fee Schedule, Final Rule. CMS-1807-F. CMS-4201-F5. https://www.govinfo.gov/content/pkg/FR-2024-12-09/html/2024-25382.htm
5. Centers for Medicare & Medicaid. CY2025 Hospital Outpatient Prospective Payment System, Final Rule: CMS-1809-FC, Addenda A, Addenda AA. https://www.cms.gov/medicare/payment/prospective-payment-systems/hospital-outpatient/regulations-notices/cms-1809-fc
6. Boston Scientific. Coding & Payment Guides. Accessed May 4, 2025. Available at: https://www.bostonscientific.com/en-US/reimbursement/interventional-cardiology.html.










