Multivessel Giant Coronary Artery Aneurysms Causing Cardiomyopathy
© 2025 HMP Global. All Rights Reserved.
Any views and opinions expressed are those of the author(s) and/or participants and do not necessarily reflect the views, policy, or position of Cath Lab Digest or HMP Global, their employees, and affiliates.
Ishan Chauhan1; Neha Gupta, MS III2; Ketul Chauhan, MD, FACC2; Sunil Gupta MD, FACC2
1Land O Lakes IB; 2Premier Heart and Vascular Center PA, Zephyrhills, Florida
The authors can be contacted via Dr. Sunil Gupta at guptas01@outlook.com.
Abstract
Giant coronary aneurysms (>8 mm) are rare entities and their management can be controversial. Only a handful of multivessel giant coronary artery aneurysms have been reported. We report the case of a 70-year-old patient who was admitted with congestive heart failure symptoms. An initial investigation discovered cardiomyopathy. Subsequently, coronary angiography and cardiac CT showed multivessel giant coronary artery aneurysms. The patient did not have signs of epicardial disease necessitating surgical intervention; therefore, the patient was treated with guideline-directed medical therapy, including oral anticoagulation and biventricular ICD implantation. We explore further management options and prognosis.
Introduction
Coronary artery ectasia is a diffuse dilation that is greater than 50% or greater than the length of the coronary artery.1 Ectasia is defined as an atherosclerosis-related remodeling phenomenon; however, a coronary artery aneurysm histologically shows an absence of tunica media, replaced with connective tissue.2 A coronary artery aneurysm is defined as a localized dilatation of a coronary artery of saccular or fusiform shape that is greater than the diameter of the normal vessel adjacent segment by 1.5 to 2 times.3 The American Heart Association has defined a giant aneurysm as having a diameter greater than 8 mm.4
Case
A 70-year-old patient presented to St. Joseph North Hospital (Tampa, Florida) cardiology service for assessment of dyspnea and elevated troponin. The patient had a previous history of mild cardiomyopathy that was managed by a primary care physician for the past few years, but had not been investigated with cardiac catheterization or an angiogram. Past medical history included obesity and hypertension. The patient was on carvedilol, rosuvastatin, losartan, and a diuretic as an outpatient. Blood pressure was 118/72 mmHg, heart rate 66, oxygen saturation 97%, and body mass index 44. On physical exam, there was visible jugular venous distention, bilateral basilar crackles, normal heart sounds S1, S2, without murmur. An abdominal exam was unremarkable. There was also bilateral 2+ pulses with 2+ bilateral edema.
Initial blood work showed a normal CBC, creatinine 1.6, CO2 18, magnesium 1.8, potassium 4.8, pro-BNP 4690, and a high sensitivity troponin trended 83, 68, and 172. Electrocardiogram showed normal sinus rhythm with left bundle branch block. A chest x-ray showed mild pulmonary congestion. An initial echocardiogram revealed an ejection fraction of 30%-35% with global hypokinesis, mild mitral regurgitation, and aortic sclerosis.
The patient was admitted with decompensated congestive heart failure for intravenous diuresis and assessment of worsening cardiomyopathy. Over next two days, the patient was started on guideline-directed medical therapy (GDMT) with intravenous diuretics, and the addition of sacubitril/valsartan and spironolactone. Once the patient was euvolemic, cardiac catheterization was performed on day 3 of hospitalization.
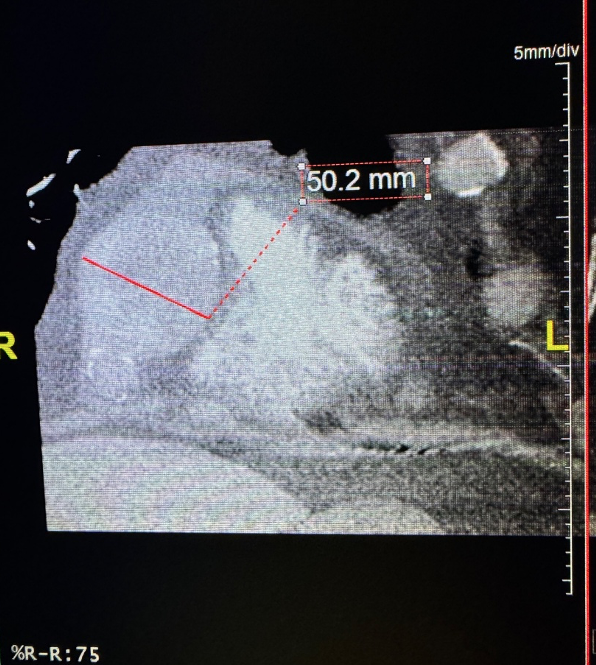
The angiogram revealed multivessel giant coronary aneurysms. The left anterior descending (LAD) coronary artery had an 11 mm proximal-to-mid artery long aneurysm involving the first diagonal branch (Figure 1). The circumflex artery had a proximal giant aneurysm measuring 24 mm, with very little visualization of distal vessel (Figure 2). Coronary dye was mostly stagnant in the coronary aneurysm, which did not fill distal vessels well. The right coronary artery (RCA) had the largest giant aneurysm, measuring 45 mm, with an inability to fill any distal vessels (Figure 3).
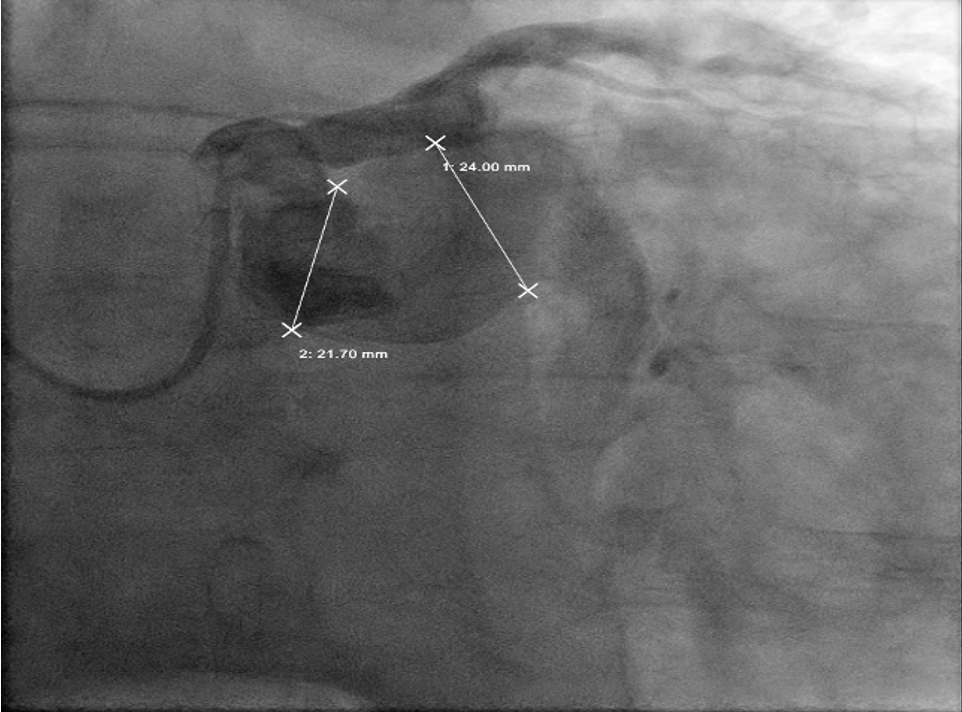
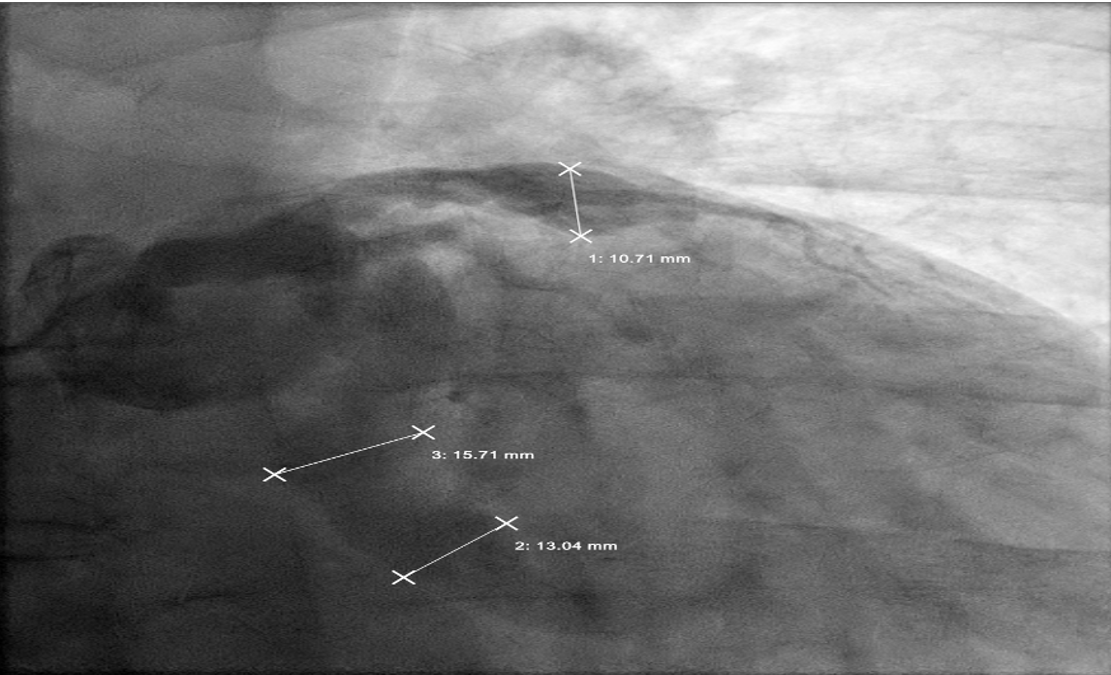
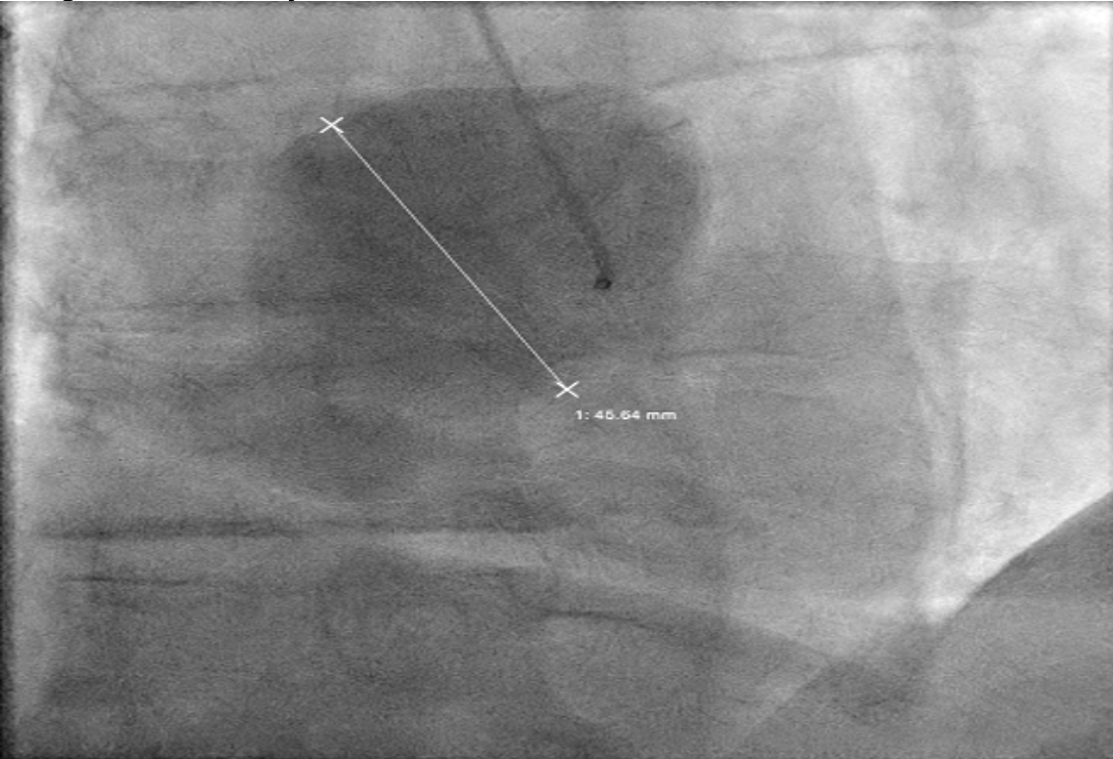
Subsequently, coronary CT angiography was performed, showing the exact sizing of the aneurysms and patency of the distal vessel, and confirming the multivessel giant coronary aneurysms diagnosis (Figures 4-6). A cardiothoracic surgery opinion was obtained, but no surgical options would be of benefit per surgical service, since there was no obstructive disease. The patient’s underlying cardiomyopathy was likely due to chronic micro thrombo-embolic phenomena from the coronary aneurysms. It was determined that the patient would be best treated with long-term oral anticoagulation and GDMT for heart failure.
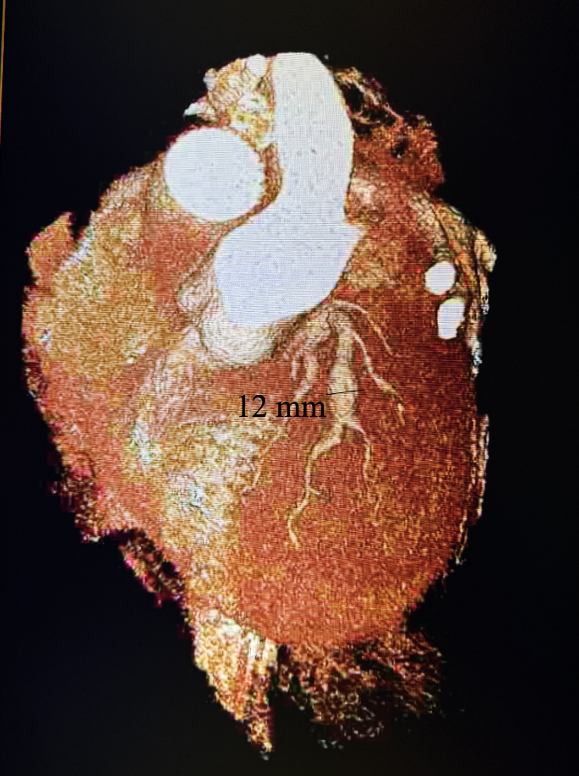
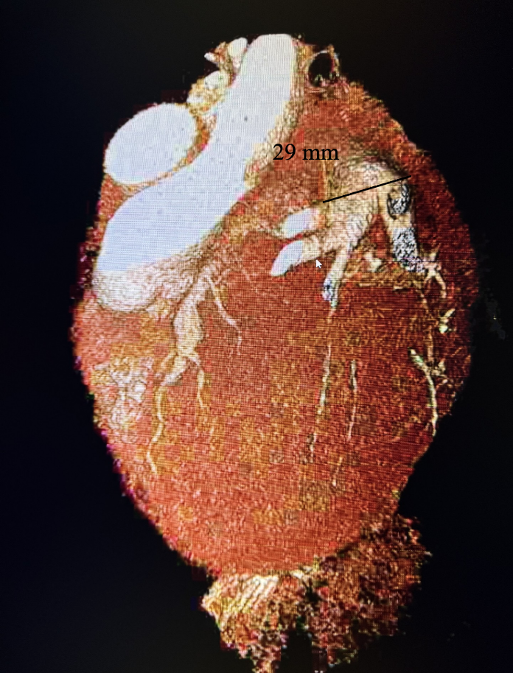
After 3 months, a repeat echocardiogram showed a persistent ejection fraction of 30%-35% with global hypokinesis. Therefore, based on American College of Cardiology (ACC) guidelines, given that the patient had underlying left bundle branch block, the patient underwent successful biventricular implantable cardioverter defibrillator (ICD) placement. At 3-month follow-up, a repeat echocardiogram showed an improvement of the patient’s symptoms to ACC Heart Failure class II with ejection fraction improving to 40%-45% with mild global hypokinesis.
Discussion
A giant coronary artery aneurysm (CAA) is a rare cardiovascular anomaly characterized by abnormal dilatation (>8 mm) of a coronary artery segment.1 Multivessel coronary aneurysm is a condition characterized by the abnormal dilatation of more than one coronary artery.3 A multivessel giant coronary artery aneurysm is a very rarely reported entity, but poses significant clinical challenges due to its association with various complications, including cardiomyopathy. CAA-related consequences, including myocardial infarction, sudden cardiac death, and heart failure, are due to thrombus formation, embolization, and a slow-flow state.5 The management of CAA is not well standardized, but there is growing interest in the potential role of oral anticoagulation therapy to mitigate thromboembolic risks.6
The etiology of CAA is varied, with atherosclerosis being the most common cause in adults. Other causes include Kawasaki disease (prevalent in pediatric populations), congenital heart defects, connective tissue disorders (such as Marfan syndrome), and inflammatory or infectious diseases (eg, syphilis, polyarteritis nodosa).7 Pathologically, CAA involves the weakening of the coronary artery wall, leading to localized dilation.
Patients with CAA may be asymptomatic or present with symptoms related to myocardial ischemia, such as chest pain, dyspnea, or signs of heart failure.8 Diagnostic modalities include coronary angiography, which is the gold standard, as well as non-invasive imaging techniques such as coronary CT angiography and cardiac MRI. These imaging tools help in assessing the size, location, and extent of the aneurysm and associated thrombus.9
The primary complications associated with CAA are thrombus formation in the aneurysm sac and embolization, leading to myocardial infarction or chronic ischemia.10 Aneurysms can disrupt normal coronary blood flow dynamics, leading to chronic myocardial ischemia and the development of ischemic cardiomyopathy.11,12
In a large study of 1356 children with Kawasaki disease who were followed for up to 15 years, coronary artery events (thrombosis, coronary artery stenosis >50% on angiography, coronary intervention, myocardial infarction, or death) were significantly more likely to occur in patients with both an absolute dimension of ≥8 mm compared to those with an aneurysm with an absolute dimension of <8 mm.13 Optimal imaging and sizing of coronary artery aneurysms is important for prognostication of future cardiovascular risk.13,14
The management of CAA lacks consensus, largely due to its rarity and the absence of large-scale randomized trials. Treatment strategies are often individualized based on the aneurysm’s size, location, associated symptoms, and presence of complications.15 Oral anticoagulation therapy has been considered for patients with CAA to reduce the risk of thromboembolic events. However, the evidence supporting the use of anticoagulation in CAA is limited and primarily based on case reports and small case series documenting successful outcomes with anticoagulation in CAA.16 These reports highlight the potential benefits but also underscore the need for careful patient selection and monitoring.6,16 Current guidelines do not provide specific recommendations for the use of oral anticoagulation in CAA due to the lack of robust evidence. Management is often guided by expert opinion and the clinical judgment of the treating physician.
Conclusion
We present a very rare case of a patient with multivessel giant coronary artery aneurysms, with likely chronic thrombo-embolic events leading to chronic ischemia and cardiomyopathy. The use of oral anticoagulation therapy presents a promising approach to managing risk, particularly with appropriate patient selection and individualization. However, due to the limited evidence base, further research is needed to establish definitive guidelines and optimize treatment strategies.
References
1. Swaye PS, Fisher LD, Litwin P, et al. Aneurysmal coronary artery disease. Circulation. 1983 Jan; 67(1): 134-138. doi:10.1161/01.cir.67.1.134
2. Nichols L, Lagana S, Parwani A. Coronary artery aneurysm: a review and hypothesis regarding etiology. Arch Pathol Lab Med. 2008 May; 132(5): 823-828. doi:10.5858/2008-132-823-CAAARA
3. Syed M, Lesch M. Coronary artery aneurysm: a review. Prog Cardiovasc Dis. 1997 Jul-Aug; 40(1): 77-84. doi:10.1016/s0033-0620(97)80024-2
4. Newburger JW, Takahashi M, Gerber MA, et al; Committee on Rheumatic Fever, Endocarditis and Kawasaki Disease; Council on Cardiovascular Disease in the Young; American Heart Association; American Academy of Pediatrics. Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Circulation. 2004 Oct 26; 110(17): 2747-2771. doi:10.1161/01.CIR.0000145143.19711.78
5. Sheikh AS, Hailan A, Kinnaird T, et al. Coronary artery aneurysm: evaluation, prognosis, and proposed treatment strategies. Heart Views. 2019 Jul-Sep; 20(3): 101-108. doi:10.4103/HEARTVIEWS.HEARTVIEWS_1_19
6. Doi T, Kataoka Y, Noguchi T, et al. Coronary artery ectasia predicts future cardiac events in patients with acute myocardial infarction. Arterioscler Thromb Vasc Biol. 2017 Dec; 37(12): 2350-2355. doi:10.1161/ATVBAHA.117.309683
7. Falsetti HL, Carrol RJ. Coronary artery aneurysm. A review of the literature with a report of 11 new cases. Chest. 1976 May; 69(5): 630-636. doi:10.1378/chest.69.5.630
8. Pahlavan PS, Niroomand F. Coronary artery aneurysm: a review. Clin Cardiol. 2006 Oct; 29(10): 439-443. doi:10.1002/clc.4960291005.
9. Matta AG, Yaacoub N, Nader V, Moussallem N, Carrie D, Roncalli J. Coronary artery aneurysm: A review. World J Cardiol. 2021 Sep 26; 13(9): 446-455. doi:10.4330/wjc.v13.i9.446
10. Hartnell GG, Parnell BM, Pridie RB. Coronary artery ectasia. Its prevalence and clinical significance in 4993 patients. Br Heart J. 1985 Oct; 54(4): 392-395. doi:10.1136/hrt.54.4.392
11. Befeler B, Aranda MJ, Embi A, et al. Coronary artery aneurysms: study of the etiology, clinical course and effect on left ventricular function and prognosis. Am J Med. 1977 Apr; 62(4): 597-607. doi:10.1016/0002-9343(77)90423-5
12. Manginas A, Cokkinos DV. Coronary artery ectasias: imaging, functional assessment and clinical implications. Eur Heart J. 2006 May; 27(9): 1026-1031. doi:10.1093/eurheartj/ehi725
13. Newburger JW, Takahashi M, Burns JC. Kawasaki disease. J Am Coll Cardiol. 2016 Apr 12; 67(14): 1738-1749. doi:10.1016/j.jacc.2015.12.073
14. Kato H, Sugimura T, Akagi T, et al. Long-term consequences of Kawasaki disease. A 10- to 21-year follow-up study of 594 patients. Circulation. 1996 Sep 15; 94(6): 1379-1385. doi:10.1161/01.cir.94.6.1379
15. Befeler B, Aranda MJ, Embi A, et al. Coronary artery aneurysms: study of the etiology, clinical course and effect on left ventricular function and prognosis. Am J Med. 1977 Apr; 62(4): 597-607. doi:10.1016/0002-9343(77)90423-5
16. D'Ascenzo F, Saglietto A, Ramakrishna H, et al; CAAR Investigators. Usefulness of oral anticoagulation in patients with coronary aneurysms: Insights from the CAAR registry. Catheter Cardiovasc Interv. 2021 Nov 1; 98(5): 864-871. doi:10.1002/ccd.29243
Find More:
Cardiovascular Ambulatory Surgery Centers (ASCs) Topic Center
Grand Rounds With Morton Kern, MD
Peripheral Artery Disease Topic Center
Podcasts: Cath Lab Conversations










