Monitoring Patients on Systemic Treatments: Deucravacitinib
© 2025 HMP Global. All Rights Reserved.
Any views and opinions expressed are those of the author(s) and/or participants and do not necessarily reflect the views, policy, or position of The Dermatologist or HMP Global, their employees, and affiliates.
Deucravacitinib (Sotyktu) is an oral selective Janus kinase (JAK) inhibitor that blocks tyrosine kinase 2 (TYK2), a mediator of signals from IL-23 and interferons α and β, thereby downregulating IL-17 production.1 Deucravacitinib was US Food and Drug Administration approved for moderate-to-severe plaque psoriasis in September 2022 and is indicated in patients who are candidates for systemic therapy or phototherapy.1 In addition to moderate-to-severe plaque psoriasis, deucravacitinib has therapeutic potential in other dermatologic conditions, such as palmoplantar pustulosis, cutaneous systemic lupus erythematosus, and Sjögren’s disease, and in non-dermatologic conditions, such as inflammatory bowel disease and psoriatic arthritis.2 Internationally, deucravacitinib has been approved for generalized pustular psoriasis and erythrodermic psoriasis.3
Q: What makes deucravacitinib different from typical JAK inhibitors?
Deucravacitinib is an allosteric inhibitor that is selective in its blockade of the JAK pathway, blocking primarily TYK2, and has a more favorable side effect profile relative to typical JAK inhibitors, which are active site inhibitors.1,4-6 Safety data from the 2-year, phase 3 POETYK PSO-1 and POETYK PSO-2 clinical trials support deucravacitinib’s tolerability with low discontinuation rates compared to apremilast and placebo.4-6 Unlike typical JAK inhibitors, deucravacitinib does not have a black box warning.
Q: Is there a routine indication for a complete blood cell count before initiation?
In the package insert, there is no indication for obtaining a complete blood cell (CBC) count with differential before initiating deucravacitinib.1 Over the 2-year, phase 3 clinical trials, there was no evidence of clinically significant neutropenia or lymphopenia with deucravacitinib treatment.5-7
Q: Is there an indication for triglyceride monitoring?
Treatment with deucravacitinib is associated with minimal increases in triglyceride levels.1 The package insert recommends periodic lipid monitoring despite no clinically relevant changes in lipids in 2-year, phase 3 safety data.1,5,6 However, it does not indicate what the frequency of lipid monitoring should be. The recommendations for typical JAK inhibitors include not only obtaining a baseline fasting lipid panel but also performing repeat testing in 8 to 12 weeks to evaluate for alterations.8-10 Lipid monitoring is recommended for the broader population, and individuals with psoriasis are at increased risk for cardiovascular disease independent of deucravacitinib treatment.11
Current lipid disorder guidelines by the US Preventive Services Task Force (USPSTF) recommend screening for lipid disorders in adults aged 40 to 75 years with no history of cardiovascular disease and at least 1 cardiovascular disease risk factor.12 In contrast, the National Institutes of Health National Heart, Lung, and Blood Institute (NIH-NHLBI) recommends patients aged 20 to 65 years should be screened once every 5 years, with men aged 45 to 65 years and women aged 55 to 65 years screened every 1 to 2 years. Additionally, the NIH-NHLBI suggests that patients over age 65 should be screened annually.13 However, clear guidelines regarding frequency of lipid screening in patients with psoriasis have not been fully elucidated.
Due to the recommendations of lipid monitoring in the general population and because patients with psoriasis are at increased risk for cardiovascular disease independent of deucravacitinib treatment, it is reasonable to obtain a baseline lipid panel then continue prudent monitoring in accordance with current clinical guidelines. In addition to lipid monitoring, patients should maintain engagement with their primary care physician for more rigorous monitoring of all cardiovascular disease risk factors.
Q: Is there an indication for routine liver function tests?
According to the package insert, patients with known or suspected liver disease should be tested with liver function tests before prescribing deucravacitinib.1 No clinically significant trends were observed in liver transaminases within clinical trials; therefore, there is no indication for routine liver function tests in patients without liver disease.5,6 Phase 3 trials do not support routine liver function tests.5,6
Liver function tests are indicated in patients who have suspected drug-induced liver injury. In the phase 3 clinical trials, 1 participant on deucravacitinib with a history of alcohol dependence and fatty liver disease developed elevations in alanine aminotransferase, aspartate aminotransferase, and bilirubin.5,6 The patient met criteria for drug-induced liver disease, and liver function tests were within physiologic limits after deucravacitinib was discontinued.5 The package insert instructs clinicians to suspend treatment with deucravacitinib until an etiology of liver injury is established.1
Q: Elevated creatine phosphokinase was reported in trials. Is this worth monitoring?
Elevated creatine phosphokinase (CPK) and rhabdomyolysis were reported in clinical trials, which led to interruption or discontinuation of deucravacitinib. In the POETYK PSO-1 and POETYK PSO-2 clinical trials, elevated serum CPK was observed, associated with physical exertion, and resolved without treatment in most cases.5,6 The package insert instructs to discontinue deucravacitinib if patients present with markedly elevated CPK or suspected myopathy. The insert states to counsel patients to promptly report muscle tenderness or weakness, especially if accompanied by fever or malaise. Routine monitoring of CPK is not indicated.
Q: Is there an indication for heightened malignancy screening?
Malignancies, including lymphomas, were observed in clinical trials.1 There are no direct indications to increase frequency of monitoring for malignancy as per the insert. Exhibited rates of nonmelanoma skin cancer (NMSC) in clinical trials were 2/531 (0.4%) and 5/833 (0.9%); among the deucravacitinib treatment group, 3 participants developed Hodgkin lymphoma, breast cancer, and hepatocellular carcinoma, respectively.5,6 The POETYK PSO-1 and PSO-2 clinical trials concluded that the overall rate of malignancy was lower than individuals with psoriasis in the Psoriasis Longitudinal Assessment and Registry and MarketScan studies.5,6 Ultimately, the deucravacitinib package insert recommends clinicians consider risks and benefits in patients with a malignancy other than NMSC. We do not recommend that patients do anything more than standard age-appropriate cancer screenings.
Q: What about infectious disease monitoring?
In phase 3 clinical trials, there were no clinically significant hematologic abnormalities to suggest profound immunosuppression or risk of opportunistic infection.5-7 Specific recommendations for infectious diseases are discussed below.
Hepatitis
According to the package insert, the impact of deucravacitinib on active hepatitis infections is unknown, as patients with positive screening for chronic hepatitis B and untreated hepatitis C were excluded from trials. The package insert advises clinicians to “consider viral hepatitis screening and monitoring for reactivation in accordance with clinical guidelines before starting therapy and during therapy with deucravacitinib.”1 Depending on the local prevalence of hepatitis, screening patients for a history of hepatitis may be sufficient; routine laboratory testing is not required. For patients with a history of or at high risk for hepatitis B or C, screening with a hepatitis panel for active or chronic hepatitis may be prudent. Initial and yearly screening may be indicated in high-risk patients with occupational exposure, those who are sexually active with multiple partners, and injection drug users.14
Herpes virus
Herpes virus reactivation was reported in clinical trials, but there are no specific screening parameters indicated in the prescribing pamphlet.1 The package insert recommends completing all age-appropriate vaccinations, including prophylactic herpes zoster vaccination. Varicella zoster vaccination (Shingrix) is approved for immunocompetent adults ages 50 years and over and in patients with a compromised immune system due to illness or treatment in patients ages 18 years and older.1 Shingrix may be administered before or after initiating treatment with deucravacitinib; however, the effectiveness of the vaccine has not been specifically characterized in patients on deucravacitinib.15 Shingrix is not a live vaccine and is safe in patients on JAK inhibitors.16 In phase 3 trials, none of the participants presented with disseminated herpes virus infection, and all resolved with antiviral therapy even while continuing deucravacitinib.9 Recommending vaccination with Shingrix seems appropriate when initiating deucravacitinib.17 With that said, we do not hold treatment with deucravacitinib in patients who decline vaccination with Shingrix.
Tuberculosis (TB)
The package insert states, “Evaluate patients for latent and active TB infection prior to initiating treatment with deucravacitinib. Do not administer deucravacitinib to patients with active TB. Initiate treatment of latent TB prior to administering deucravacitinib.”1 This is commonly interpreted as an indication to test for latent TB and screen for active TB. The insert does not recommend routine annual TB testing; rather, it advises clinicians to monitor patients for the symptomatology of active TB, such as fever, cough, night sweats, and unexplained weight loss.
The USPSTF published recommendations regarding screening asymptomatic adults at increased risk for latent TB infection. Screening for latent TB, as per the USPSTF, is indicated in patients in high-risk categories, such as individuals who travel to endemic areas, face homelessness, were formerly incarcerated, or work in health care settings.18,19 Both the tuberculin skin test and the interferon-gamma release assay (IGRA), such as QuantiFERON-TB Gold In-Tube, can be used interchangeably for screening latent TB infection.18-20 Neither the USPSTF nor the prescribing information specifies an interval or criteria for repeat TB testing. However, in high-risk patients, the tuberculin skin test or IGRA repeated annually may be prudent.
Key Takeaways
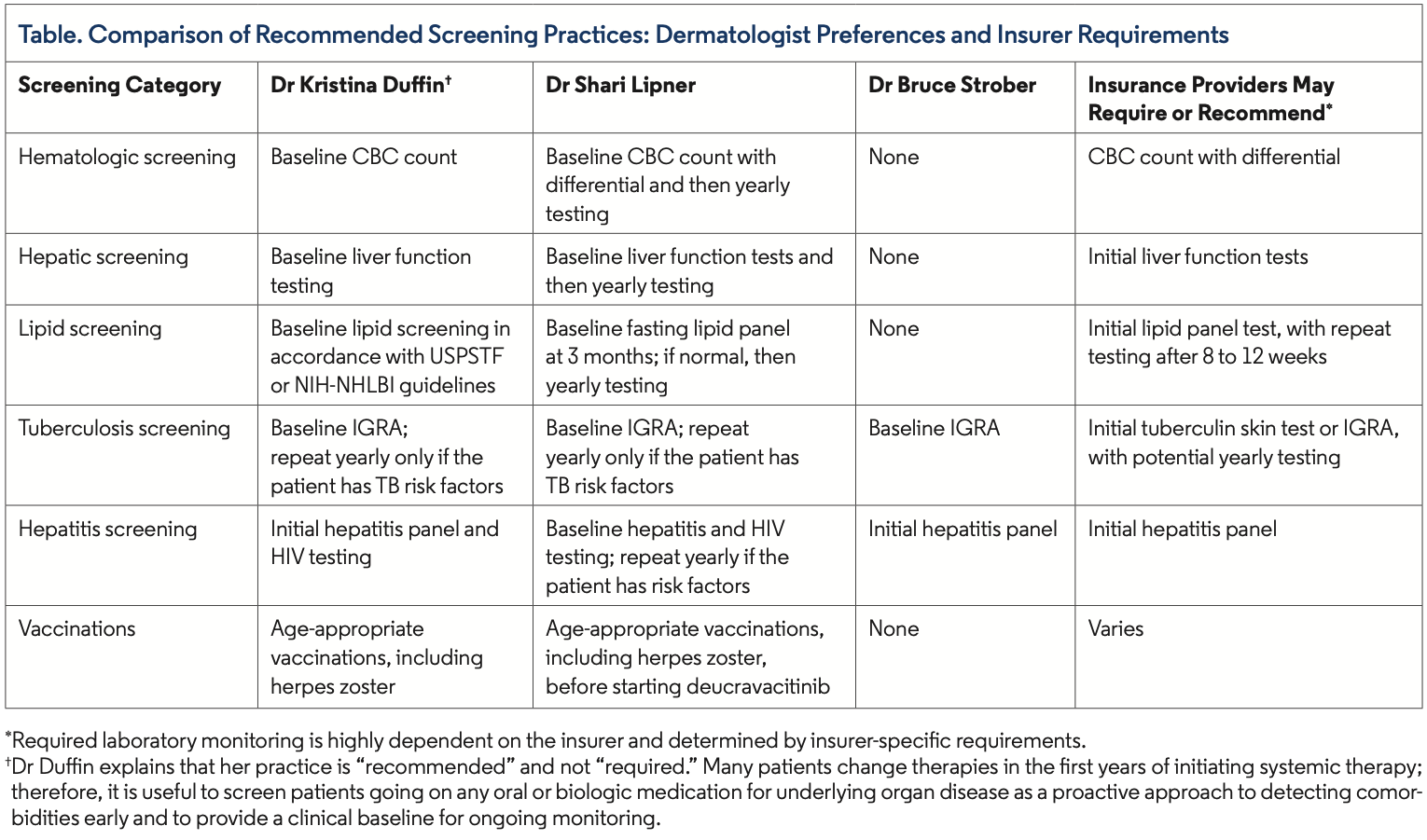
Clinicians vary in how they monitor patients on deucravacitinib, and insurers may require further monitoring or testing, with specific requirements varying by insurer and location (Table). Patient specific needs, including TB exposure, hepatitis exposure, and malignancy, may affect your decision to monitor.
Dr Collins is an internal medicine resident at UMass Chan Medical School in Worcester, MA. Dr Huang is a post-doctoral research fellow in the department of dermatology at Wake Forest School of Medicine in Winston-Salem, NC. Dr Duffin is a professor, chair of the department of dermatology, and recently appointed interim dean of the Spencer Fox Eccles School of Medicine at the University of Utah in Salt Lake City, UT. Dr Lipner is an associate professor of clinical dermatology, associate attending physician, and director of the nail division at New York-Presbyterian Hospital/Weill Cornell Medical Center in New York, NY. Dr Strober is a clinical professor of dermatology at Yale University School of Medicine in New Haven, CT, and a board-certified dermatologist at Central Connecticut Dermatology in Cromwell, CT. Dr Feldman is a professor of dermatology, pathology, and social sciences and health policy at Wake Forest University School of Medicine in Winston-Salem, NC, and the chief medical editor of The Dermatologist.
Disclosures: Dr Duffin has received honoraria for consulting from AbbVie, Amgen, Boehringer Ingelheim, Bristol Myers Squibb, Janssen Pharmaceuticals, Novartis, Pfizer, and UCB. She has received research grants (paid as salary) from Boehringer Ingelheim, Pfizer, and UCB and owns stock in AbbVie and UCB.
Dr Strober has been a consultant for AbbVie, Almirall, Alumis, Amgen, Apogee, Arcutis, Boehringer Ingelheim, Bristol Myers Squibb, CorEvitas, Dermavant, Eli Lilly and Company, Immunovant, Janssen Pharmaceuticals, Leo Pharma, Maruho, Meiji Seika Pharma, Neurocrine, Novartis, Oruka Therapeutics, Pfizer, Protagonist, UCB, Rapt, Regeneron, Sanofi Genzyme, SelectION, and Takeda. He has been a speaker for AbbVie, Arcutis, Eli Lilly and Company, Incyte, Johnson & Johnson, Regeneron, and Sanofi Genzyme. He has received research grants from CorEvitas, Incyte, Oruka Therapeutics, and Takeda and holds stock options in Mindera Health and SelectION.
Dr Feldman has received research, speaking, and/or consulting support from AbbVie, Accordant, Almirall, Alumis, Alvotech, Amgen, Arcutis, Arena, Argenx, Biocon, Boehringer Ingelheim, Bristol Myers Squibb, Caremark, Celgene, Dermavant, Eli Lilly and Company, Eurofins, Forte, Galderma, GlaxoSmithKline Stiefel, Helsinn, Informa Healthcare, Janssen Pharmaceuticals, Leo Pharma, Menlo, Merck & Co., Micreos, Mylan, National Biological Corporation, National Psoriasis Foundation, Novartis, Ono Pharma, Ortho Dermatology, Oruka Therapeutics, Pfizer, Qurient, Regeneron, Samsung, Sanofi, Sun Pharma, Teladoc, UCB, UpToDate, Verrica, and vTv Therapeutics. He is founder and part owner of Causa Research and holds stock in Sensal Health.
References
- SOTYKTU (deucravacitinib) medication guide. Bristol Myers Squibb. September 2022. Accessed May 3, 2025. https://packageinserts.bms.com/medguide/medguide_sotyktu.pdf
- Bang CH, Park CJ, Kim YS. The expanding therapeutic potential of deucravacitinib beyond psoriasis: a narrative review. J Clin Med. 2025;14(5):1745. doi:10.3390/jcm14051745
- Hoy SM. Deucravacitinib: first approval. Drugs. 2022;82(17):1671-1679. doi:10.1007/s40265-022-01796-y
- New four-year Sotyktu (deucravacitinib) data demonstrate durable responserates and consistent safety in moderate-to-severe plaque psoriasis. Bristol Myers Squibb. May 16, 2024. Accessed September 9, 2025. https://news.bms.com/news/details/2024/New-Four-Year-Sotyktu-deucravacitinib-Data-Demonstrate-Durable-Response-Rates-and-Consistent-Safety-in-Moderate-to-Severe-Plaque-Psoriasis/default.aspx
- Armstrong AW, Gooderham M, Warren RB, et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, placebo-controlled phase 3 POETYK PSO-1 trial. J Am Acad Dermatol. 2023;88(1):29-39. doi:10.1016/j.jaad.2022.07.002
- Strober B, Thaçi D, Sofen H, et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, phase 3 Program fOr Evaluation of TYK2 inhibitor psoriasis second trial. J Am Acad Dermatol. 2023;88(1):40-51. doi:10.1016/J.JAAD.2022.08.061
- Lebwohl M, Warren RB, Sofen H, et al. Deucravacitinib in plaque psoriasis: 2-year safety and efficacy results from the phase III POETYK trials. Br J Dermatol. 2024;190(5):668-679. doi:10.1093/bjd/ljae014
- Haag C, Alexis A, Aoki V, et al. A practical guide to using oral Janus kinase inhibitors for atopic dermatitis from the International Eczema Council. Br J Dermatol. 2024;192(1):135-143. doi:10.1093/bjd/ljae342
- Samuel C, Cornman H, Kambala A, Kwatra SG. A review on the safety of using JAK inhibitors in dermatology: clinical and laboratory monitoring. Dermatol Ther (Heidelb). 2023;13(3):729-749. doi:10.1007/s13555-023-00892-5
- Paolino G, Valenti M, Carugno A, et al. Serum lipids alterations in patients under systemic JAK inhibitors treatments in dermatology: clinical aspects and management. Medicina (Kaunas). 2025;61(1):54. doi:10.3390/medicina61010054
- Egeberg A, Skov L, Joshi AA, et al. The relationship between duration of psoriasis, vascular inflammation, and cardiovascular events. J Am Acad Dermatol. 2017;77(4):650-656.e3. doi:10.1016/j.jaad.2017.06.028
- Statin use for the primary prevention of cardiovascular disease in adults: Preventive medication. US Preventive Services Task Force. August 23, 2022. Accessed September 9, 2025. https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/statin-use-in-adults-preventive-medication
- Blood cholesterol: diagnosis. National Heart, Lung, and Blood Institute. April 18, 2024. Accessed September 9, 2025. https://www.nhlbi.nih.gov/health/blood-cholesterol/diagnosis
- Viral hepatitis surveillance and case management. Centers for Disease Control and Prevention. February 29, 2024. Accessed September 9, 2025. https://www.cdc.gov/hepatitis/statistics/surveillanceguidance/index.htm
- Package insert—Shingrix. US Food and Drug Administration. Revised July 2025. Accessed September 9, 2025. https://www.fda.gov/files/vaccines,%20blood%20&%20biologics/published/Package-Insert-SHINGRIX.pdf
- Esteban-Vazquez A, Steiner M, Castañeda E, et al. The real-world study of immunogenicity and safety of the adjuvant recombinant vaccine against varicella zoster virus in patients with immune-mediated inflammatory diseases treated with Janus kinase inhibitors. Vaccines (Basel). 2023;11(10):1610. doi:10.3390/vaccines11101610
- Baumrin E, Van Voorhees A, Garg A, Feldman SR, Merola JF. A systematic review of herpes zoster incidence and consensus recommendations on vaccination in adult patients on systemic therapy for psoriasis or psoriatic arthritis: from the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol. 2019;81(1):102-110. doi:10.1016/j.jaad.2019.03.017
- US Preventive Services Task Force; Mangione CM, Barry MJ, Nicholson WK, et al. Screening for latent tuberculosis infection in adults: US Preventive Services Task Force recommendation statement. JAMA. 2023;329(17): 1487-1494. doi:10.1001/jama.2023.4899
- Lewinsohn DM, Leonard MK, LoBue PA, et al. Official American Thoracic Society/Infectious Diseases Society of America/Centers for Disease Control and Prevention clinical practice guidelines: diagnosis of tuberculosis in adults and children. Clin Infect Dis. 2017;64(2):111-115. doi:10.1093/cid/ciw778
- Mazurek GH, Jereb J, Vernon A, LoBue P, Goldberg S, Castro K. Updated guidelines for using interferon gamma release assays to detect Mycobacterium tuberculosis infection—United States, 2010. MMWR Recomm Rep. 2010;59(RR05):1-25.