Precision Under the Surface: Electrical Impedance Spectroscopy for Pigmented Lesion Evaluation
Pigmented skin lesions remain a diagnostic dilemma even for experienced dermatologists. In many cases, the clinical features of benign lesions, such as melanocytic nevi, lentigines, and pigmented seborrheic keratoses, may be concerning and resemble those of melanoma. On the other hand, some melanomas may lack overtly concerning features, or these features may be subtle, leading to a delay in biopsy and therefore diagnosis. The goal is to detect melanoma at its earliest stage, including those lesions with severe dysplasia that may progress to melanoma if untreated, while avoiding unnecessary biopsies of benign skin lesions.
Routine evaluation of pigmented lesions in dermatology relies on visual inspection using the ABCD criteria and, for many clinicians, dermoscopy to assess symmetry, structure, color distribution, and vascular patterns. These tools are foundational to melanoma detection and remain central to daily practice. However, they are inherently limited to features that can be appreciated at or near the skin surface.
In clinically ambiguous lesions, the challenge is rarely a lack of experience or vigilance. Rather, it is the absence of biologic information below the epidermis, where early malignant transformation may involve architectural and cellular disruption before reproducible surface features emerge. In such cases, morphology alone may not fully characterize underlying behavior.
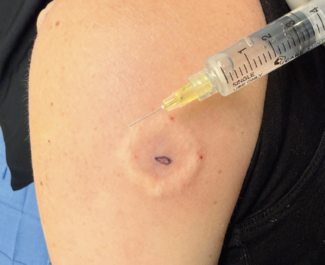
Electrical impedance spectroscopy (EIS) extends lesion assessment beyond what can be seen. By measuring electrical properties that reflect tissue organization and heterogeneity below the skin surface, such as electrical resistance differences between benign and malignant cells, EIS provides an objective diagnostic signal that is fundamentally different from visual or optical evaluation.
The US Food and Drug Administration (FDA)-approved device Nevisense uses EIS and augmented intelligence (AI) to detect melanoma. Although Nevisense has only recently been introduced into US clinical practice following FDA approval, it has been CE marked and used routinely across Europe since 2013. This history of sustained real-world use provides important context when considering its role in contemporary dermatology.
In this discussion, Dr Stephen Hess shares how he incorporates Nevisense into the evaluation of clinically ambiguous pigmented lesions to enhance early detection and strengthen diagnostic confidence when surface findings alone are insufficient.
Stephen D. Hess, MD, PhD, is a board-certified dermatologist and the owner, president, and medical director of Center City Dermatology, with office locations in Philadelphia and Exton, PA. He completed his dermatology residency at the Hospital of the University of Pennsylvania, as well as a 1-year fellowship in cutaneous oncology. He has a doctorate in immunology and is also board-certified in micrographic and dermatologic surgery. Dr Hess maintains a faculty appointment as a clinical associate in dermatology at the Hospital of the University of Pennsylvania.
The Dermatologist: How did you first decide to incorporate Nevisense into your diagnostic workflow for pigmented lesions?
Dr Hess: When I first considered incorporating Nevisense, I reviewed the clinical evidence supporting its use. The data supporting FDA approval were derived from one of the largest prospective melanoma studies conducted in this area, which immediately stood out.1 The combination of that evidence base and more than a decade of routine clinical use in Europe was compelling. I was also impressed by one particular clinical study, which demonstrated improved diagnostic accuracy among dermatology practitioners with differing experience levels.2 Nevisense has demonstrated the ability to increase both sensitivity and specificity compared to the standard combination of visual assessment plus dermoscopy while reducing the number needed to biopsy, which is an important metric for melanoma detection.
I thought carefully about how it would integrate across my practice. We work closely with physician assistants, and achieving consistency in evaluating ambiguous lesions can be difficult. Having access to an objective data point that assesses tissue below the skin surface has helped support more uniform evaluations while preserving clinical judgment.
Patients have responded positively as well. Many appreciate knowing that additional, noninvasive, AI-driven, objective data are being applied when lesions are difficult to assess. Improved diagnostic accuracy for early melanoma detection while potentially avoiding unnecessary biopsies is appealing to my patients. The incorporation of Nevisense into my practice has become a distinguishing feature of how we approach melanoma evaluation.
The Dermatologist: Where does Nevisense fit in your clinical workflow?
Dr Hess: I use Nevisense as part of my initial assessment when evaluating pigmented lesions that raise concern but do not meet clear criteria for removal. Clinical examination and dermoscopy remain central, and Nevisense adds subsurface biologic information that complements what I am seeing at the surface.
In lesions with subtle asymmetry, focal atypia, or discordant features, the EIS data help contextualize surface findings. Having that information available during the visit allows me to synthesize clinical, dermoscopic, and subsurface inputs into a more complete assessment of risk. The device is not designed to assess lesions that are clearly benign or those with obvious clinical features of malignancy.
An important advantage is that Nevisense can provide true point-of-care assessment. The EIS analysis is done in real-time, generating a score between 0 and 10, which directly correlates with the likelihood of the lesion being malignant. This allows for timely and informed clinical decisions rather than delayed reassessment.
From a practical standpoint, Nevisense also integrates well operationally. The clinician must decide when to use Nevisense, but the procedure itself can be delegated to and performed by appropriately trained ancillary staff members. There is an established CPT (current procedural terminology) code that allows the assessment to be incorporated into routine workflows without requiring special arrangements.
The Dermatologist: How has your use of Nevisense affected how you think about next steps in clinically ambiguous lesions?
Dr Hess: Nevisense adds objective risk stratification in cases that are clinically challenging. I interpret the EIS score alongside the full clinical picture, including patient risk factors, family history, lesion evolution, and surface features. It is one piece of the assessment, not a stand-alone determinant.
The EIS scoring ranges are helpful in framing biologic risk. Lower scores (0–3) are associated with a high negative predictive value, on the order of approximately 99%, which is reassuring in the correct clinical context. As EIS scores increase, the likelihood of dysplasia and melanoma increases in direct proportion.
Over time, observing how these scores correlate with histopathologic findings has been particularly informative. In my practice, an EIS score of 4 or higher raises concern and may tip the balance toward intervention (biopsy or complete lesion removal), although decisions are always individualized.
The Dermatologist: In what types of lesions or patient cases is Nevisense most informative?
Dr Hess: It has been particularly valuable in higher-risk patients, including those with a personal or family history of melanoma, significant sun damage, or a high burden of clinically atypical nevi (dysplastic nevus syndrome). In these settings, subsurface information can help distinguish true biologic risk from low-grade background atypia based on visual assessment.
Some of my patients may be hesitant to consent to a biopsy, especially if the lesion is in a cosmetically sensitive area, if they are “needle adverse” or “needle phobic,” or of they simply have “biopsy fatigue.” In these patients, a higher Nevisense EIS score provides objective data that may help me convince them to proceed with a biopsy. In other anxious patients, or parents concerned about a pigmented lesion on their child’s skin, a lower Nevisense score may allow me to reassure them that the lesion is extremely unlikely to be malignant and therefore can be followed clinically.
The Dermatologist: Does Nevisense ever change your clinical decision-making?
Dr Hess: Nevisense can influence my clinical decision-making when evaluating challenging pigmented lesions. In lesions with subtle or discordant surface features, subsurface biologic information can raise suspicion earlier than morphology alone, indicating the need for intervention.
I have had many cases where a lesion appeared relatively unremarkable initially, yet the Nevisense output suggested underlying pathology that justified earlier intervention. In that sense, it has helped identify melanoma earlier in its evolution.
In other cases, reassuring Nevisense data have raised my threshold for intervention in clinically ambiguous pigmented lesions. A low Nevisense score may allow a lesion to be followed clinically rather than removing it during the visit, depending on other risk factors and clinical judgment. I always emphasize to my patients that they must continue to monitor clinically ambiguous lesions for any changes if they are not removed, regardless of the Nevisense results.
The Dermatologist: How do you balance the device’s output with your own expertise?
Dr Hess: Over time, correlating Nevisense EIS scores with pathology has built confidence in how the output aligns with histologic outcomes. Clinical history, visual assessment, and dermoscopy remain foundational. Nevisense adds subsurface biologic information that complements those inputs rather than competing with them.
In some cases, having access to these data has allowed for earlier identification of lesions with concerning behavior than might have been apparent from surface features alone. It is the integration of experience, clinical assessment, and objective subsurface data that strengthens decision-making.
The Dermatologist: Have you observed any impact on melanoma detection in your practice?
Dr Hess: In my practice, Nevisense has enhanced diagnostic accuracy for melanoma. Since implementing Nevisense, I have detected melanomas in patients for whom I would have otherwise decided to defer biopsy. I have also detected melanomas in a number of patients who initially refused intervention but agreed to have a biopsy after a concerning Nevisense score was noted. My physician assistants have all had similar experiences. An important point is that each of these melanomas was a clinically ambiguous lesion detected early (in situ or T1a lesions). Early detection of melanoma, when it is treatable and potentially curable, is one of the most important things we do in dermatology, and Nevisense has enhanced my ability to achieve this goal in my practice.
The Dermatologist: Are there limitations clinicians should understand?
Dr Hess: It is important to understand what Nevisense is designed to do and what it is not. I view it as a point-of-care diagnostic adjunct intended to add objective insight to lesion assessment, particularly to reduce the risk that early melanoma is missed. It is not a stand-alone diagnostic. It is meant to be used by dermatologists and dermatology providers; it is not recommended for use by primary care providers.
Proper training is essential. All providers and staff must complete FDA-required training, and supervised use is important to ensure consistent technique and reliable data.
There are also defined technical limitations. Nevisense is not indicated for acral or mucosal sites and should not be used in the presence of surface fluid or active dermatitis. Hair must be shaved or trimmed so that it does not interfere with the electrical signal. The device cannot be used in lesions less than 2 mm, those associated with a scar, or on pigmented lesions within a tattoo.
Finally, the EIS score represents one component of a comprehensive assessment. Lower scores are associated with a high negative predictive value, whereas higher scores reflect increasing biologic concern. How that information is applied must always be guided by the full clinical picture.
Used thoughtfully and within its intended parameters, Nevisense adds meaningful information at the point in lesion assessment where clinical judgment is most challenged.
The Dermatologist: How do you see the role of technology in skin cancer care evolving?
Dr Hess: I believe that Nevisense may eventually become the standard of care for assessment of challenging pigmented skin lesions. Nevisense supports earlier melanoma detection in select cases without altering established standards of care. Over time, correlating these assessments with pathology will continue to reinforce confidence in the value of integrating subsurface information into routine lesion evaluation. Similar to the role of gene expression profiling to assess skin cancer prognosis and guide treatment decisions, Nevisense fits into the broader landscape of using technology and AI in the field of dermatology and medicine in general.
Incorporating Nevisense into my practice has been one of the best decisions I have made as a clinician and business owner. Improving diagnostic accuracy and early melanoma detection is a goal shared by both clinicians and patients. I would encourage my peers to consider adding this valuable tool to their practice.
Disclosure: Dr Hess is a paid consultant for SciBase.
References
1. Malvehy J, Hauschild C, Curiel-Lewandrowski C, et al. Clinical performance of the Nevisense system in cutaneous melanoma detection: an international, multicentre, prospective and blinded clinical trial on efficacy and safety. Br J Dermatol. 2014;171(5):1099-1107. doi:10.1111/bjd.13121
2. Litchman G, Teplitz R, Svoboda RM, Del Rosso JQ. Increased uniformity in diagnostic accuracy of pigmented lesions using electrical impedance spectroscopy information. J Clin Aesthet Dermatol. 2021;14(10):35-36.