Association Between Peak Anti-Xa Activity and Bleeding Events in Patients Treated With NOACs
A Meta-Analysis of Observational Studies
A Meta-Analysis of Observational Studies
Key Summary
- The authors conducted a meta-analysis of 4 observational studies that compared peak anti-Xa levels in adults treated with apixaban or rivaroxaban.
- Bleeding was associated with significantly higher peak anti-Xa levels, which were consistent across drug subgroups; meta-regression found no significant clinical predictors.
- Anti-Xa monitoring is not guideline-recommended but may aid risk stratification in select high-risk patients. Observational design, assay variability, and heterogeneity limit inference; prospective validation is needed.
© 2026 HMP Global. All Rights Reserved.
Any views and opinions expressed are those of the author(s) and/or participants and do not necessarily reflect the views, policy, or position of Vascular Disease Management or HMP Global, their employees, and affiliates.
VASCULAR DISEASE MANAGEMENT. 2026;23(1):E8-E14.
Abstract
Background: While novel oral anticoagulants (NOACs) are widely used for stroke prevention in atrial fibrillation, bleeding remains a major concern. Anti-Xa activity monitoring has emerged as a potential tool to guide individualized risk stratification. Objective: To assess the association between peak anti-Xa levels and bleeding events in patients treated with apixaban or rivaroxaban through a meta-analysis of observational studies. Methods: A systematic review identified 4 studies including 1087 patients (24.4% with bleeding). Peak anti-Xa levels were compared between patients with and without bleeding events using a random-effects meta-analysis. Results: Patients with bleeding had significantly higher peak anti-Xa levels (+36.7 ng/mL; 95% confidence interval: 29.9-43.5; P < .001), a finding consistent across drug-specific subgroups. No significant clinical predictors of bleeding were identified via meta-regression. Conclusion: Elevated peak anti-Xa activity is associated with increased bleeding risk in patients treated with NOACs. Although not currently recommended in routine practice, anti-Xa monitoring may help refine bleeding risk assessment in selected high-risk individuals. Prospective studies are needed to validate its clinical utility.
Introduction
In recent years, novel oral anticoagulants (NOACs) have significantly transformed the management of atrial fibrillation, markedly reducing cardioembolic risk, even in older adult and frail patients who were traditionally excluded from anticoagulant therapy. Despite their proven safety and efficacy, supported by large clinical trials, NOACs require lifelong use, often in aging patients with declining renal function and fluctuating body weight. Therefore, careful risk stratification using validated scoring systems is essential to enable personalized treatment, including reduced dosages when appropriate.
The increasing availability of specific reversal agents has further enhanced the safety profile of NOACs, contributing to the growing use of anti-Xa level monitoring. Anti-Xa measurement played a key role in the development of direct oral anticoagulants, particularly in phase II trials to define optimal dosing and guide phase III study design. While not used routinely in practice, anti-Xa testing is increasingly available and reserved for specific scenarios: major bleeding, urgent high-risk surgery, suspected overdose or nonadherence, severe renal impairment, extreme body weight, unknown drug interactions, and before administering reversal agents such as andexanet alfa. In these contexts, it helps assess anticoagulation intensity and inform clinical decisions.
The purpose of this research was to evaluate whether peak Anti-Xa value was associated with bleeding events in observational studies.
Methods
Four studies1-4 were identified in the literature as reporting anti-Xa levels in patients treated with apixaban and rivaroxaban. Data were systematically extracted from these studies (1 retrospective and 3 prospective), which reported anti-Xa values in both bleeding and nonbleeding patients. Key clinical variables at the study level were collected from a total population of 1087 patients, of whom 213 (24.4%) experienced a bleeding event. For each study, mean peak anti-Xa levels, standard deviations, and sample sizes were obtained. Meta-analyses were performed using random-effects models. Heterogeneity was assessed using the I² statistic and further explored through meta-regression, funnel plots, and subgroup analyses. Anti-Xa levels were also correlated with relevant clinical variables from the included studies.
Results
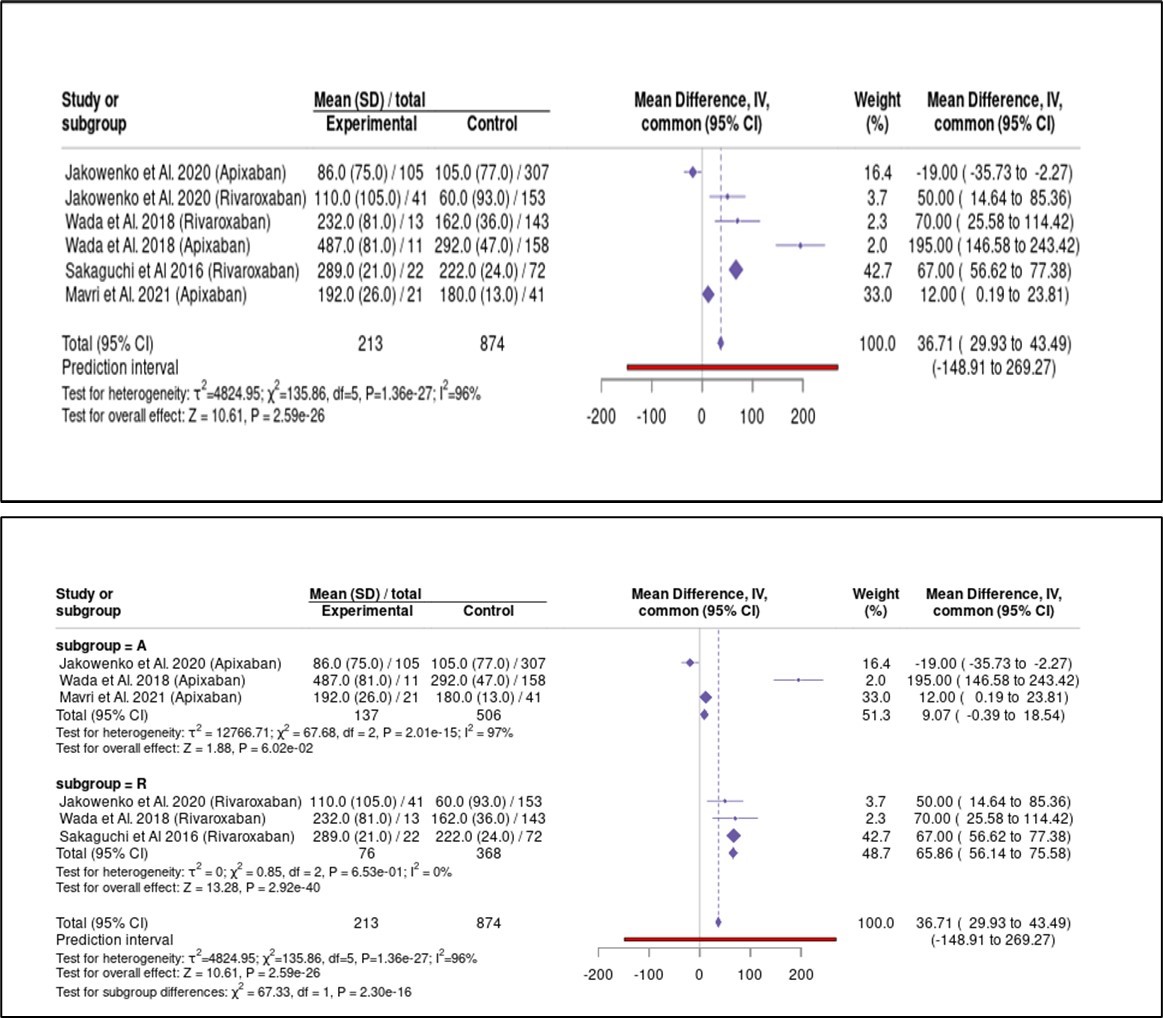
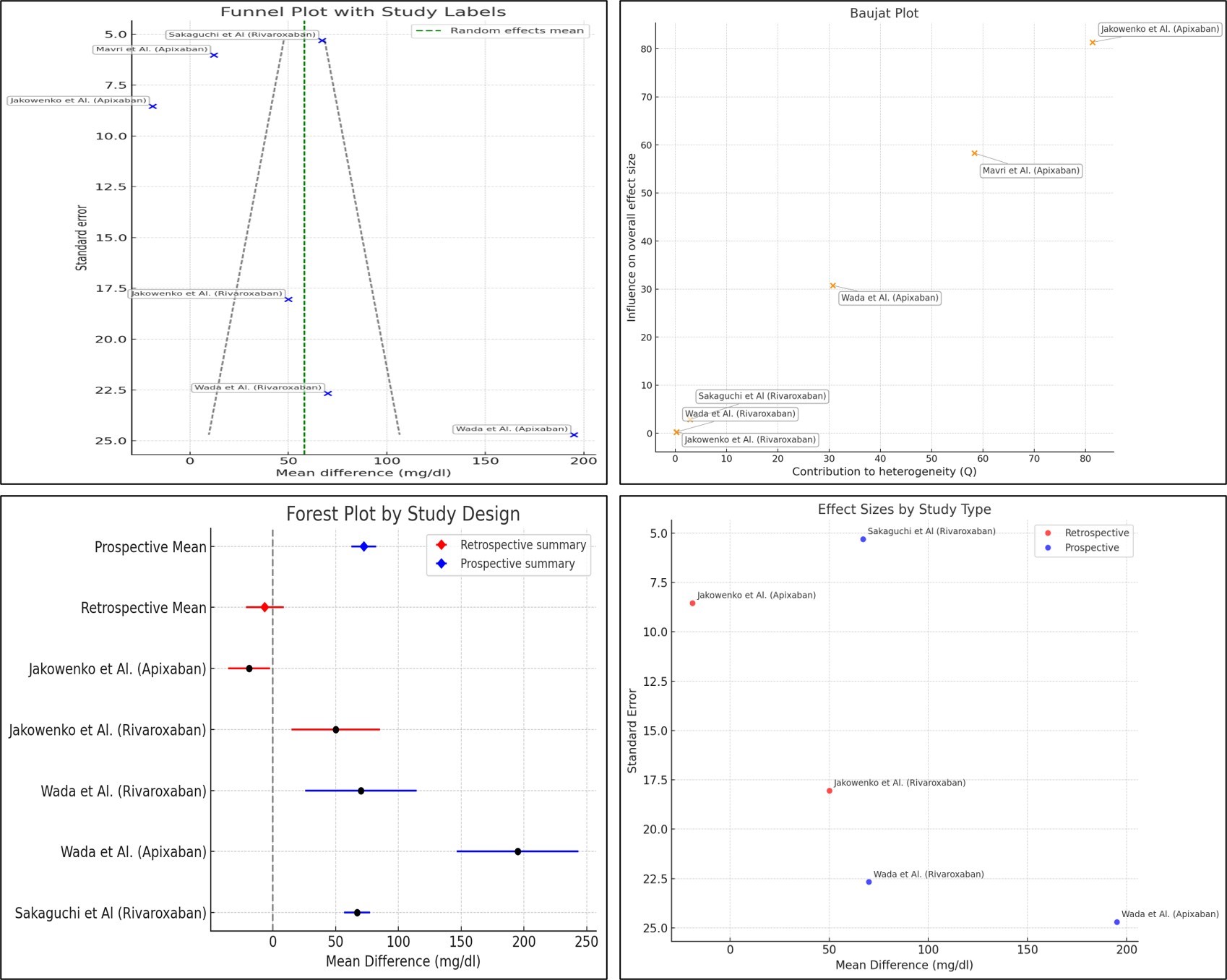
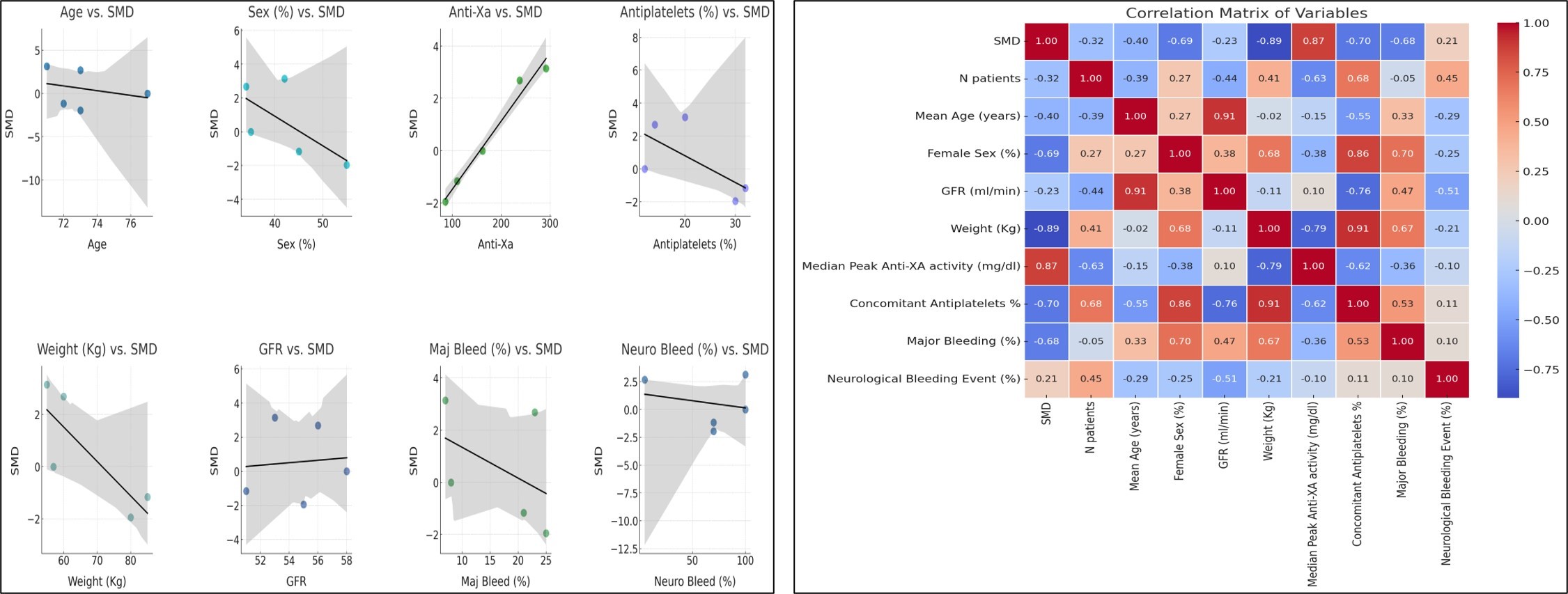
The main clinical characteristics of the included studies are summarized in the Table. Patients who experienced bleeding events had significantly higher mean anti-Xa values compared to those without bleeding. This difference remained statistically significant in both the apixaban and rivaroxaban subgroups. The pooled mean difference in peak anti-Xa activity was +36.7 ng/mL (95% confidence interval [CI]: 29.9-43.5, P < .001), indicating substantially higher levels in patients with bleeding events (Figure 1). Funnel plots showed a relatively symmetrical distribution, though a few smaller studies displayed more extreme effect sizes, suggesting the limited risk of publication bias. The Baujat plot identified 2 studies (Wada et al and Sakaguchi et al) as major contributors to both heterogeneity and overall influence (Figure 2). When interpreting this heterogeneity, it is important to consider the pharmacokinetic differences between apixaban and rivaroxaban. Rivaroxaban, administered once daily, is associated with higher peak and lower trough levels compared to apixaban, which is dosed twice daily and produces more stable concentrations. Meta-regression analysis revealed no statistically significant predictors among age, sex, estimated glomerular filtration rate, body weight, or antiplatelet therapy (all P > .05). However, trends suggested that female sex and lower body weight may be potential modifiers of anti-Xa levels and bleeding risk (Figure 3).
Table 1. Main clinical characteristics of the studies included in the meta-analysis.
| Study | N patients | Type of study | Mean age (years) | Female sex (%) | GFR (mL/min) | Weight (kg) | Median anti-XA activity (mg/dL) | Concomitant antiplatelets (%) | Major bleeding (%) |
Neurological bleeding event (%) |
| Jakowenko et al (apixaban) | 412 | Retrospective | 73 | 55 | 55 | 80 | 86 | 30 | 25 | 70 |
| Jakowenko et al (rivaroxaban) | 194 | Retrospective | 72 | 45 | 51 | 85 | 110 | 32 | 21 | 70 |
| Wada et al (rivaroxaban) | 156 | Prospective | 77 | 35 | 58 | 57 | 162 | 12 | 8 | 100 |
| Wada et al (apixaban) | 169 | Prospective | 71 | 42 | 53 | 55 | 292 | 20 | 7 | 100 |
| Sakaguchi et al (rivaroxaban) | 94 | Prospective | 73 | 34 | 56 | 60 | 238 | 14 | 23 | 5 |
| Mavri et al (apixaban) | 62 | Retrospective | 78 | 60 | 63 | 75 | 192 | - | 34 | 5 |
Abbreviation: GFR, glomerular filtration rate.
Discussion
This study supports a positive association between elevated peak anti-Xa activity and bleeding events in patients treated with NOACs, specifically apixaban and rivaroxaban. This association remained statistically significant within both apixaban and rivaroxaban subgroups. The pooled mean difference in peak anti-Xa activity was +36.7 ng/mL (95% CI: 29.9-43.5; P < .001), suggesting a clinically relevant relationship. Although anti-Xa monitoring is not currently recommended by international guidelines, our findings suggest the potential utility of this biomarker in refining bleeding risk stratification.
The mean difference in anti-Xa levels between bleeding and nonbleeding patients was statistically significant and remained consistent across sensitivity analyses within drug-specific subgroups despite the pharmacokinetic differences of apixaban and rivaroxaban. Anti-Xa measurement may complement validated bleeding risk scores and clinical markers, particularly in scenarios requiring dose adjustment or closer monitoring, such as patients with borderline renal function or extreme body weight. While promising, these findings require confirmation in larger, prospective cohorts.
Given the clinical impact and health care costs associated with bleeding, anti-Xa monitoring could prove cost-effective if shown to reduce events through individualized risk modulation. However, such evaluation lies beyond the scope of this study and would require a randomized trial.5 Several limitations must be considered. First, heterogeneity in patient populations and laboratory methodologies across included studies may introduce bias. Also, assay kits calibrated for individual NOACs hinder direct comparison between drugs and studies. Despite applying a random-effects model, residual confounding cannot be excluded. Moreover, all studies were observational, limiting causal inference.
Another limitation is the definition of hemorrhagic events not consistently assessable due to classification heterogeneity. Anti-Xa dosing may only be meaningful when aimed at preventing major bleeding. Nonetheless, these findings contribute to the ongoing discussion on dynamic biomarkers in anticoagulation. As the anticoagulated population continues to age and present with multiple comorbidities, static risk scores may be insufficient to capture evolving bleeding risk.
Anti-Xa monitoring may offer valuable granularity in selected scenarios, especially when considering alternatives such as left atrial appendage occlusion or transitioning to emerging agents such as factor XI inhibitors under phase III investigation. Further large-scale prospective studies are needed to validate these findings, define meaningful thresholds, and explore integration into personalized anticoagulation strategies (Figure 4).
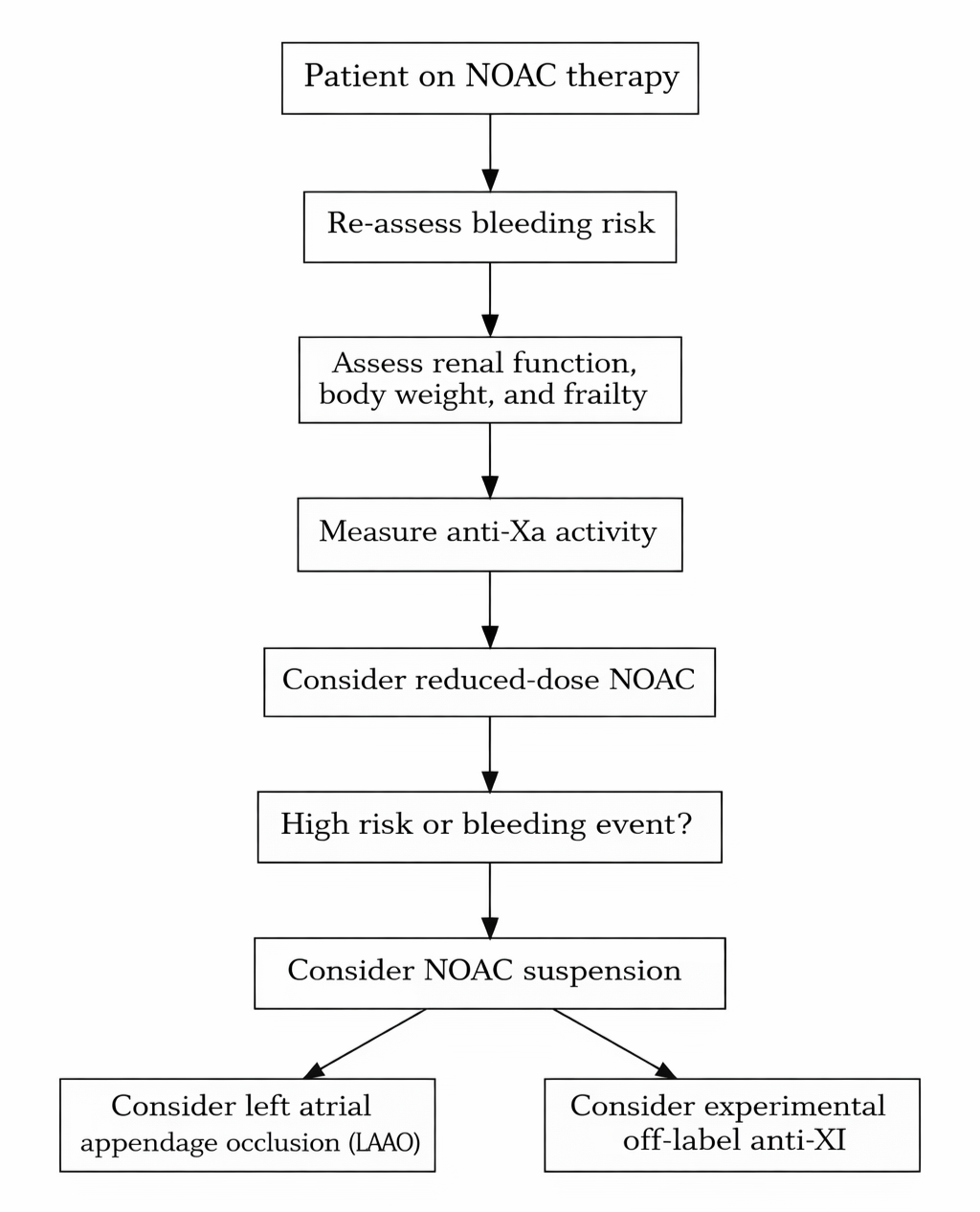
Abbreviation: NOAC, novel oral anticoagulant.
Conclusion
This meta-analysis supports a positive association between elevated anti-Xa activity and bleeding risk in patients treated with NOACs. Although not currently part of routine clinical practice, our findings suggest that anti-Xa measurement could enhance bleeding risk assessment in select high-risk populations. Potential biases limit this perspective, underscoring the need for large-scale prospective validation to clarify clinical utility. In the context of an aging and increasingly frail population requiring life-long anticoagulation, anti-Xa monitoring may offer added value in refining bleeding risk stratification and guiding decisions for emerging alternatives such as left atrial appendage closure and investigational factor XI inhibitors.
Affiliations and Disclosures
Alberto Francesco Cereda, MD, Marco Stracqualursi, MD, Antonio Gabriele Franchina, MD, Lorenzo Tua, MD, and Stefano Lucreziotti, MD, are from the Cardiology Unit at ASST Santi Paolo e Carlo, Milan, Italy; Federico Biondi, MD, is from IRCCS San Raffaele, Milan, Italy; and Marco Biolcati, MD, is from Cardiology 4–Diagnostic and Rehabilitation Cardiology, ASST Grande Ospedale Metropolitano Niguarda, Milan, Italy.
The authors report no financial relationships or conflicts of interest regarding the content herein.
Manuscript accepted December 4, 2025.
Address for Correspondence: Albert Francesco Cereda, MD, Cardiology Unit, ASST Santi Paolo e Carlo, Via Antonio di Rudinì, 8, 20142 Milano MI, Italy. E-mail: tskcer@hotmail.it
References
1. Jakowenko N, Nguyen S, Ruegger M, Dinh A, Salazar E, Donahue KR. Apixaban and rivaroxaban anti-Xa level utilization and associated bleeding events within an academic health system. Thromb Res. 2020;196:276-282. doi:10.1016/j.thromres.2020.09.002
2. Wada S, Toyoda K, Sato S, Matsuki T, et al. Anti-Xa activity and event risk in patients with direct factor Xa inhibitors initiated early after stroke. Circ J. 2018;82(11):2872-2879. doi:10.1253/circj.CJ-18-0506
3. Sakaguchi T, Osanai H, Murase Y, et al. Monitoring of anti-Xa activity and factors related to bleeding events: a study in Japanese patients with nonvalvular atrial fibrillation receiving rivaroxaban. J Cardiol. 2017;70(3):244-249. doi:10.1016/j.jjcc.2016.11.013
4. Mavri A, Vene N, Božič-Mijovski M, et al. Apixaban concentration variability and relation to clinical outcomes in real-life patients with atrial fibrillation. Sci Rep. 2021;11(1):13908. doi:10.1038/s41598-021-93372-9
5. Moner-Banet T, Alberio L, Bart P. Does one dose really fit all? On the monitoring of direct oral anticoagulants: a review of the literature. Hamostaseologie. 2020;40(2):184-200. doi:10.1055/a-1113-0655